|
|
| Line 1: |
Line 1: |
| − | [[wikipedia: Advection and Groundwater Flow | Advection and Groundwater Flow]] is a probable human carcinogen that is present in the environment due to historic use as a solvent and current use as a precursor in chemical synthesis. TCP is mobile and highly persistent in soil and groundwater. TCP is not currently regulated at the national level in the United States, but maximum contaminant levels (MCLs) have been established or are proposed at the state level in Hawaii, California, and New Jersey. Treatment of TCP contamination using conventional groundwater and soil treatment methods is either ineffective or costly, but alternative remediation strategies are under development. | + | [[wikipedia: Advection | Advection]] is the movement of groundwater through the subsurface due to pressure and gravitational energy. The combined processes of [[wikipedia: Advection | advection]], [[wikipedia: Dispersion | dispersion]], [[wikipedia: Diffusion | diffusion]], sorption, and degradation control how long a contaminant plume will grow, remain stable, or shrink, as well as how easy or difficult it will be to remediate. The combined effects of these processes are represented in solute transport models with the advection-dispersion-reaction equation. |
| − | <div style="float:right;margin:0 0 2em 2em;">__TOC__</div>
| + | |
| | '''Related Article(s):''' | | '''Related Article(s):''' |
| − | *[[Soil & Groundwater Contaminants]] | + | *[[Dispersion and Diffusion]] |
| | | | |
| | + | <div style="float:right;margin:0 0 2em 2em;">__TOC__</div> |
| | + | <br /> |
| | | | |
| − | '''Contributor(s):''' [[Dr. Alexandra Salter-Blanc]] | + | '''CONTRIBUTOR(S):''' [[Dr. Charles Newell, P.E.]] |
| | | | |
| | | | |
| | '''Key Resource(s):''' | | '''Key Resource(s):''' |
| − | *[https://serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Emerging-Issues/ER-1457/ER-1457/(language)/eng-US Prospects for Remediation of Advection and Groundwater Flow by Natural and Engineered Abiotic Degradation Reactions]<ref name= "Tratnyek2010P">Tratnyek, P.G., Sarathy, V., Salter, A.J., Nurmi, J.T., Johnson, G.O., DeVoe, T., Lee, P., 2010. Prospects for Remediation of Advection and Groundwater Flow by Natural and Engineered Abiotic Degradation Reactions. Project ER-1457. [https://serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Emerging-Issues/ER-1457/ER-1457/(language)/eng-US Report pdf]</ref> | + | *[http://www.wiley.com/WileyCDA/WileyTitle/productCd-0471597627.html Physical and Chemical Hydrogeology]<ref name="D&S1998">Domenico, P.A. and Schwartz, F.W., 1998. Physical and chemical hydrogeology. John Wiley & Sons, 2nd Ed., 528 pgs. ISBN 978-0-471-59762-9.</ref> |
| − | | |
| − | ==Introduction==
| |
| − | [[File:SalterBlanc-Article2-Figure1.jpg|thumbnail|300px|left|Figure 1. Ball and stick representation of TCP (courtesy of the [http://www.ebs.ieh.ohsu.edu/tratnyek Tratnyek Research Group]).]]
| |
| − | [[wikipedia: Advection and Groundwater Flow | Advection and Groundwater Flow]] is a man-made chemical that was used in the past primarily as a solvent and extractive agent, a paint and varnish remover, and as a cleaning and degreasing agent (Fig. 1)<ref name= "ATSDR1992"> Agency for Toxic Substances and Disease Registry, 1992. Toxicological Profile for Advection and Groundwater Flow. [[Media:atsdr_toxprofile_stp57.pdf|Report pdf]]</ref>. Currently, TCP is primarily used in chemical synthesis (e.g., synthesis of polysulfone liquid polymers used in the aerospace and automotive industries; [[wikipedia:: Hexafluoropropylene | hexafluoropropylene]] used in the agricultural, electronic, and pharmaceutical industries; [[wikipedia:: Polysulfide | polysulfide]] polymers used as sealants in manufacturing and construction; and [[wikipedia: 1,3-Dichloropropene | 1,3-dichloropropene]] used in agriculture as a soil fumigant)<ref name= "ATSDR1992" /><ref name= "CH2MHILL2005"> CH2M HILL, 2005. Interim Guidance for Investigating Potential Advection and Groundwater Flow Sources in San Gabriel Valley Area 3. [[Media:INTERIM_GUIDANCE_FOR_INVESTIGATING_POTENTIAL_1%2C2%2C3-TRICHLOROPROPANE_SOURCES.pdf|Report pdf]]</ref>. TCP may be present in products containing these chemicals as an impurity<ref name= "ATSDR1992" /><ref name= "CH2MHILL2005" />. For example, the 1,2-dichlropropane/1,3-dichlropropene soil fumigant mixture (trade name D-D), which is no longer sold in the United States, contained TCP as an impurity and has been linked to TCP contamination in groundwater<ref>Oki, D.S.,Giambelluca, T.W., 1987. DBCP, EDB, and TCP contamination of ground water in Hawaii. Ground Water, 25(6), 693-702. [https://doi.org/10.1111/j.1745-6584.1987.tb02210.x doi:10.1111/j.1745-6584.1987.tb02210.x]</ref><ref name= "CH2MHILL2005" />. Soil fumigants composed primarily of 1,3-dichlropropene, which are currently in use, may also contain TCP as an impurity (e.g., Telone II is reported to contain up to 0.17 percent TCP by weight<ref name= "Kielhorn2003">Kielhorn, J., G. Könnecker, C. Pohlenz-Michel, S. Schmidt, and I. Mangelsdorf, 2003. Advection and Groundwater Flow. Geneva: World Health Organization. Concise International Chemical Assessment, Document 56. [http://www.who.int/ipcs/publications/cicad/en/cicad56.pdf Report pdf]</ref>).
| |
| − | TCP contamination is problematic because it is "reasonably anticipated to be a human carcinogen" based on evidence of carcinogenicity to animals<ref>National Toxicology Program, 2016. Report on Carcinogens, 14th ed. U.S. Department of Health and Human Services, Public Health Service. [http://ntp.niehs.nih.gov/pubhealth/roc/index-1.html Report pdfs]</ref>. Toxicity to humans appears to be high relative to other chlorinated solvents<ref name= "Kielhorn2003" />, suggesting that even low-level exposure to TCP could pose a significant human health risk.
| |
| | | | |
| − | ==Environmental Fate, Occurrence, and Regulation== | + | ==Groundwater Flow== |
| − | ===Environmental Fate===
| + | Groundwater will flow from areas of high [[wikipedia: Hydraulic head | hydraulic head]] (pressure and gravitational energy) to areas of lower hydraulic head (Fig. 1). The slope of the change in hydraulic head is known as the hydraulic gradient. If groundwater is flowing and contains dissolved contaminants it can transport the contaminants from areas with high hydraulic head to lower hydraulic head (or “downgradient”). |
| − | [[File:SalterBlanc-Article2-Table1.jpg|thumbnail|650px|right|Table 1. Physical and chemical properties of TCP<ref name= "USEPA2014">United States Environmental Protection Agency, 2014. Technical Fact Sheet—Advection and Groundwater Flow. EPA 505-F-14-007. [[Media:1_2_3_TCP_fact_sheet.pdf|Fact Sheet pdf]]</ref>. See also ASTDR (1992)<ref name= "ATSDR1992" /> and Dombeck and Borg (2005)<ref>Dombeck, G., Borg., C., 2005. Multicontaminant Treatment for 1,2,3 Trichloropropane Destruction Using the HiPOx Reactor.” Proceedings of the 2005 National Groundwater Association Conference on MTBE and Perchlorate: Assessment, Remediation, and Public Policy with permission of the NGWA Press. ISBN 1-56034-120-3 </ref>.]] | + | [[File:Newell-Article 1-Fig1.JPG|thumbnail|center|500px|Figure 1. Hydraulic gradient (typically described in units of m/m or ft/ft) is the difference in hydraulic head from Point A to Point B (ΔH) divided by the distance between them (ΔL). In unconfined aquifers, the hydraulic gradient can also be described as the slope of the water table (Adapted from course notes developed by Dr. R.J. Mitchell, Western Washington University).]] |
| | | | |
| − | TCP’s fate in the environment is governed by its physical and chemical properties (Table 1). TCP is not anticipated to sorb strongly to soil making it likely to leach into groundwater and exhibit high mobility. In addition, TCP is moderately volatile and can partition from surface water and moist soil into the atmosphere. Because TCP is only slightly soluble and denser than water, it is capable of forming a [[wikipedia: Dense non-aqueous phase liquid | dense non-aqueous phase liquid (DNAPL)]]. TCP is generally resistant to [[wikipedia:: Biodegradation | biodegradation]], [[wikipedia: Hydrolysis | hydrolysis]], oxidation, and reduction under naturally occurring conditions making it highly persistent in the environment<ref name= "Tratnyek2010P"/>.
| + | ==Darcy's Law== |
| | + | In unconsolidated geologic settings (gravel, sand, silt, and clay) and highly fractured systems, the rate of groundwater movement can be expressed using [[wikipedia: Darcy's law | Darcy’s Law]]. This law is a fundamental mathematical relationship in the groundwater field and can be expressed this way: |
| | | | |
| − | ===Occurrence===
| + | [[File:Newell-Article 1-Equation 1rr.jpg|center|500px]] |
| − | TCP has been detected in ~1% percent of United States Geological Survey tested public supply and domestic well samples. More specifically, TCP was detected in 1.2% of public supply well samples collected between 1993 and 2007<ref name= "Toccalino 2010">Toccalino, P.L., Norman, J.E., Hitt, K.J., 2010. Quality of source water from public-supply wells in the United States, 1993-2007. U. S. Geological Survey. Scientific Investigations Report 2010-5024. [[Media:Quality_of_source_water_from_public-supply_wells_in_the_United_States%2C_1993-2007.pdf|Report pdf]]</ref> and 0.66% of domestic supply well samples collected between 1991 and 2004<ref name= "DeSimone2009">DeSimone, L.A., 2009. Quality of water from domestic wells in principal aquifers of the United States, 1991–2004: U.S. Geological Survey. Scientific Investigations Report 2008–5227. [[Media:Quality_of_Water_from_Domestic_wells.pdf|Report pdf]]</ref>. TCP was detected at a higher rate in domestic supply well samples associated with agricultural land-use studies than samples associated with aquifer studies (3.5% versus 0.2%)<ref name= "DeSimone2009" />.
| + | ::Where: |
| | + | :::Q = Flow per area (Volume groundwater flow per area per time, such as m<sup>3</sup>/yr) |
| | + | :::A = Cross sectional area perpendicular to groundwater flow (length<sup>2</sup>, such as m<sup>2</sup>) |
| | + | :::V<sub>D</sub> = “Darcy Velocity”; another way to describe groundwater flow as the flow per unit area (units of length per time, such as ft/yr) |
| | + | :::K = Hydraulic Conductivity (sometimes called “permeability”) (length per time) |
| | + | :::ΔH = Difference in hydraulic head between two lateral points (length) |
| | + | :::ΔL = Length between two lateral points (length) |
| | | | |
| − | ===Regulation===
| + | [https://en.wikipedia.org/wiki/Hydraulic_conductivity Hydraulic conductivity] (Table 1 and Fig. 2) is a measure of how easy groundwater flows through a porous medium, or alternatively, how much energy it takes to force water through a porous medium. For example, fine sand (sand with small grains) means smaller pores and more frictional resistance and therefore lower hydraulic conductivity (Fig. 2) compared to coarse sand (sand with large grains), which has less resistance to flow. |
| − | The United States Environmental Protection Agency (EPA) has not established a MCL for TCP, although guidelines and health standards are in place<ref name= "USEPA2014" />. TCP was included in the Contaminant Candidate List 3<ref name= "EPA2009">United States Environmental Protection Agency (US EPA), 2009. Drinking water contaminant candidate list 3-final. Federal Register 74(194), 51850–51862. [[Media:drinking_water_contaminant_candidate_list.pdf|Report pdf]]</ref> and the Unregulated Contaminant Monitoring Rule 3 (UCMR 3)<ref name= "EPA2009" />. The UCMR 3 specified that data be collected on TCP occurrence in public water systems over the period of January 2013 to December 2015 against a reference concentration range of 0.0004 to 0.04 μg/L<ref name= "USEPA2016">United States Environmental Protection Agency, 2016. The Third Unregulated Contaminant Monitoring Rule (UCMR 3): Data Summary. EPA 815-S-16-004. [[Media:third_unregulated_contaminant_monitoring_rule_july_2016.pdf|Report pdf]]</ref>. The reference concentration range was determined based on a cancer risk of 10<sup>-6</sup> to 10<sup>-4</sup><ref name= "USEPA2016" /> and derived from an oral slope factor of 30 mg/kg-day determined for the EPA’s Integrated Risk Information System<ref>United States Environmental Protection Agency and Integrated Risk Information System, 2009. Advection and Groundwater Flow (CASRN 96-18-4). [[Media:123-TRICHLOROPROPANE_ERD.PDF|Report pdf]]</ref>. Available partial UCMR 3 data published as of July 2016 indicate that TCP has been detected above the UCMR-specified minimum reporting level of 0.03 μg/L at 1.3% of monitored public water systems<ref name= "USEPA2016" />.
| + | [[File:Newell-Article 1-Table1r.jpg|550px|thumbnail|center|Table 1. Representative values of total porosity (n), effective porosity (n<sub>e</sub>), and hydraulic conductivity (K) for different aquifer materials<ref name="D&S1998"/><ref>McWhorter, D.B. and Sunada, D.K., 1977. Ground-water hydrology and hydraulics. Water Resources Publication, LLC. 304 pgs. ISBN 978-0-918334-18-3 </ref><ref>Freeze, R.A. and Cherry, J.A., 1979. Groundwater. 604 pgs. ISBN 978-0133653120</ref>.]] |
| | + | [[File:Newell-Article 1-Fig2.jpg|475px|thumbnail|center|Figure 2. Hydraulic conductivity of selected rocks<ref>Heath, R.C., 1983. Basic ground-water hydrology, U.S. Geological Survey Water-Supply Paper 2220, 86 pgs. [[Media:Heath-1983-Basic_groundwater_hydrology_water_supply_paper.pdf|Report pdf]]</ref>.]] |
| | | | |
| − | At the state level, Hawaii is the only state that has established a MCL for TCP in drinking water. This MCL is 0.6 μg/L<ref>Hawaii Department of Health, 2013. Amendment and Compilation of Chapter 11-20 Hawaii Administrative Rules. September 5. [[Media:Amendment_and_Compilation_of_Chapter_11-20_Hawaii_Administrative_Rules.pdf|Report pdf]]</ref>. California has established a notification level of 0.005 μg/L and a public health goal of 0.0007 μg/L<ref>Office of Environmental Health and Hazard Assessment, California Environmental Protection Agency (OEHHA), 2009. Public Health Goals for Advection and Groundwater Flow in Drinking Water. August.</ref>. The state is currently in the process of developing a MCL for TCP and a preliminary recommendation of 0.005 μg/L was made public July 2016. Up-to-date information can be found at the [http://www.waterboards.ca.gov/drinking_water/certlic/drinkingwater/123TCP.shtml California Environmental Protection Agency State Water Resources Control Board]. In October 2016, the New Jersey Drinking Water Quality Institute recommended proposition and adoption of a 0.03-μg/L MCL based on a practical quantitation limit of the same value (a health based MCL of 0.0005 μg/L was recommended<ref>New Jersey Drinking Water Quality Institute, 2016. Maximum contaminant level recommendations for 1,2,3-trichloroporpane in drinking water. [[Media:Maximum_contaminant_level_recommendations_for_1%2C2%2C3-trichloroporpane_in_drinking_water.pdf|Report pdf]]</ref>).
| + | <BR CLEAR="left">Darcy’s Law was first described by Henry Darcy (1856)<ref>Darcy, H., 1856. Les Fontaines Publiques de la Ville de Dijon, Dalmont, Paris. [https://doi.org/10.1029/2001wr000727 doi: 10.1029/2001WR000727]</ref> in a report regarding a water supply system he designed for the city of Dijon, France. He ran experiments and concluded that the amount of water flowing through a closed tube of sand (dark grey box in Figure 3) depends on (a) the change in the hydraulic head between the inlet and outlet of the tube, and (b) the hydraulic conductivity of the sand in the tube. Groundwater flows rapidly in the case of higher pressure (ΔH) and permeable materials such as gravel or coarse sand, but flows slowly when the pressure is lower and low-permeability material such as fine sand or silt. |
| | | | |
| − | ==Prospects for Remediation== | + | [[File:Newell-Article 1-Fig3..JPG|500px|thumbnail|right|Figure 3. Conceptual explanation of Darcy’s Law based on Darcy’s experiment (Adapted from course notes developed by Dr. R.J. Mitchell, Western Washington University).]]<BR CLEAR="left">Since Darcy’s time, Darcy’s Law has been adapted to calculate the actual velocity that the groundwater is moving in units such as meters traveled per year. This quantity is called “interstitial velocity” or “seepage velocity” and is calculated by dividing the Darcy Velocity (flow per unit area) by the actual open pore area where groundwater is flowing, the “effective porosity” (Table 1): |
| | + | [[File:Newell-Article 1-Equation 2r.jpg|400px]]<br /> |
| | + | :Where: |
| | + | ::V<sub>S</sub> = “interstitial velocity” or “seepage velocity” (units of length per time, such as m/sec)<br /> |
| | + | ::V<sub>D</sub> = “Darcy Velocity”; another way to describe groundwater flow as the flow per unit area (units of length per time)<br /> |
| | + | ::n<sub>e</sub> = Effective porosity (unitless) |
| | | | |
| − | ===Physical Removal===
| + | Effective porosity is smaller than total porosity. The difference is that total porosity includes some dead-end pores that do not support groundwater. Typically values for total and effective porosity are shown in Table 1. |
| − | TCP contamination in drinking water is typically treated using granular activated carbon (GAC)<ref>TetraTech, 2012. Report to the Hawaii Department of Health, Safe Drinking Water Branch, Regarding the Human Health Risks of Advection and Groundwater Flow in Tap Water.</ref><ref>Ozekin, K., 2016. Advection and Groundwater Flow State of the Science. Water Research Foundation. [[Media:1%2C2%2C3-Trichloropropane_state_of_science.pdf|Report pdf]]</ref>. GAC is also applied for groundwater treatment in pump and treat scenarios<ref>California Environmental Protection Agency, 2016. State Water Resources Control Board. Groundwater Information Sheet: Advection and Groundwater Flow. [[Media:waterboards.ca.govgamadocscoc_tcp123.pdf|Report pdf]]</ref>. However, based on published [[wikipedia: Freundlich equation | Freundlich adsorption isotherm]] parameters<ref>Snoeyink, V.L., Summers, R.S, 1990. Adsorption of Organic Compounds, In: Pontius, F.W. (ed.). Water Quality and Treatment. New York, NY. McGraw-Hill.</ref>, less TCP mass is adsorbed per gram of carbon compared to other volatile organic compounds (VOCs) such as [[wikipedia: Tetrachloroethylene | tetrachloroethene]] and [[wikipedia:: Trichloroethylene | trichloroethene]]. This results in an increased carbon usage rate and treatment cost compared to these contaminants. Air stripping, air sparging, and soil vacuum extraction are also less effective compared to other VOCs because of TCP’s relatively low [[wikipedia:: Henry's law | Henry’s Law]] constant.
| |
| | | | |
| − | ===Transformation=== | + | ==Darcy Velocity and Seepage Velocity== |
| − | [[File:SalterBlanc-Article2-Figure2.jpg|thumbnail|650px|left|Figure 2. Summary of anticipated, primary reaction pathways for degradation of TCP. Oxidation, hydrolysis, and hydrogenolysis are represented by the horizontal arrows. Elimination (dehydrochlorination) and reductive elimination are shown with vertical arrows. [O] represents oxygenation (by oxidation or hydrolysis), [H] represents reduction. Gray indicates products that appear to be of lesser significance. Reprinted with permission from Sarathy et al. (2010)<ref name= "Sarathy2010" /> (Copyright 2010, American Chemical Society).]]
| + | In groundwater calculations, Darcy Velocity and Seepage Velocity are two different things used for different purposes. For any calculation where the actual flow rate in units of volume per time (such as liters per day or gallons per minute) is involved, then the original Darcy Equation should be used (calculate V<sub>D</sub>; Equation 1) without using effective porosity. When calculating solute travel time, then the seepage velocity calculation (V<sub>S</sub>; Equation 2) must be used and an estimate of effective porosity is required. Figure 4 illustrates the differences between Darcy Velocity and Seepage Velocity. |
| − | Potential TCP degradation pathways include hydrolysis, oxidation, and reduction (Fig. 2). These overall pathways are expected to be similar for abiotic and biotic reactions<ref name= "Sarathy2010">Sarathy, V., Salter, A.J., Nurmi, J.T., O’Brien Johnson, G., Johnson, R.L., Tratnyek, P.G., 2010. Degradation of 1, 2, 3-trichloropropane (TCP): Hydrolysis, elimination, and reduction by iron and zinc. Environmental Science & Technology, 44(2), 787-793. [https://doi.org/10.1021/es902595j doi:10.1021/es902595j]</ref>, but the rates of the reactions (and their resulting significance) are dependent on natural and engineered conditions.
| |
| | | | |
| − | Hydrolysis of TCP is negligible under ambient pH and temperature conditions, but is favorable at high pH and/or temperature<ref name= "Tratnyek2010P" /><ref name= "Sarathy2010" />. For example, ammonia gas can be used to raise soil pH and stimulate alkaline hydrolysis of chlorinated propanes including TCP<ref>Medina, V.F., S.A. Waisner, C. Coyle, C. Griggs, M. Maxwell, 2016. Laboratory-Scale Demonstration Using Dilute Ammonia Gas-Induced Alkaline Hydrolysis of Soil Contaminants (Chlorinated Propanes and Explosives). ERDC/EL TR-16-10. [[Media:ERDC_EL_TR_16_10.pdf|Report pdf]]</ref>. In situ thermal remediation may also produce favorable conditions for TCP hydrolysis<ref name= "Tratnyek2010P"/><ref name= "Sarathy2010" />. Oxidation using mild/specific oxidants like [[wikipedia:: Permanganate | permanganate]] is negligible, but stronger oxidants like those used in [[In Situ Chemical Oxidation - ISCO|in situ chemical oxidation (ISCO)]] (especially hydroxyl and sulfate radicals) do oxidize TCP<ref name= "Tratnyek2010P" />. Reduction by naturally occurring reductants (i.e., those associated with natural attenuation) is negligible and granular [[Zerovalent Iron (ZVI)| zerovalent iron (ZVI)]] produces only slow reduction<ref name= "Tratnyek2010P"/><ref name= "Sarathy2010" />. Nano-scale ZVI and palladized ZVI produce faster reduction, but the reaction is not anticipated to be fast enough to be useful in typical remediation applications<ref name= "Sarathy2010" />. Commercial-grade zerovalent zinc (ZVZ) reduces TCP relatively quickly under a range of laboratory and field conditions to produce propene without significant accumulation of intermediates<ref name= "Sarathy2010" /><ref>Salter-Blanc, A.J., Tratnyek, P.G., 2011. Effects of solution chemistry on the dechlorination of 1, 2, 3-trichloropropane by zero-valent zinc. Environmental Science & Technology, 45(9), 4073-4079. [https://doi.org/10.1021/es104081p doi:10.1021/es104081p]</ref><ref>Salter‐Blanc, A.J., Suchomel, E.J., Fortuna, J.H., Nurmi, J.T., Walker, C., Krug, T., O'Hara, S., Ruiz, N., Morley, T., Tratnyek, P.G., 2012. Evaluation of Zerovalent Zinc for Treatment of 1, 2, 3‐Trichloropropane‐Contaminated Groundwater: Laboratory and Field Assessment. Groundwater Monitoring & Remediation, 32(4), 42-52. [https://doi.org/10.1111/j.1745-6592.2012.01402.x doi:10.1111/j.1745-6592.2012.01402.x]</ref>.
| + | [[File:Newell-Article 1-Fig4.JPG|550px|thumbnail|center|Figure 4. Difference between Darcy Velocity (also called Specific Discharge) and Seepage Velocity (also called Interstitial Velocity).]] |
| | | | |
| − | Aerobic biodegradation is not favorable as no naturally-occurring microorganisms have been identified that degrade TCP under aerobic conditions<ref name= "Samin2012">Samin, G., Janssen, D.B., 2012. Transformation and biodegradation of 1, 2, 3-trichloropropane (TCP). Environmental Science and Pollution Research, 19(8), 3067-3078. [https://doi.org/10.1007/s11356-012-0859-3 doi:10.1007/s11356-012-0859-3]</ref>. Relatively slow aerobic cometabolism by the ammonia oxidizing bacterium [[wikipedia:: Nitrosomonas europaea | ''Nitrosomonas europaea'']] and other populations has been reported<ref>Vannelli, T.O.D.D., Logan, M.Y.K.E., Arciero, D.M., Hooper, A.B., 1990. Degradation of halogenated aliphatic compounds by the ammonia-oxidizing bacterium Nitrosomonas europaea. Applied and Environmental Microbiology, 56(4), 1169-1171. [http://aem.asm.org/content/56/4/1169.short Journal Article]</ref><ref name= "Samin2012" /> and genetic engineering has been used to develop organisms capable of utilizing TCP as a sole carbon source under aerobic conditions<ref>Bosma, T., Damborský, J., Stucki, G., Janssen, D.B., 2002. Biodegradation of 1, 2, 3-trichloropropane through directed evolution and heterologous expression of a haloalkane dehalogenase gene. Applied and Environmental Microbiology, 68(7), 3582-3587. [https://doi.org/10.1007/s002530051263 doi: 10.1007/s002530051263]</ref><ref name= "Samin2012" />. Anaerobic reductive dechlorination has been reported in enrichment cultures<ref>Löffler F.E., J.E. Champine, K.M Ritalahti, S.J. Sprague, J.M. Tiedje, 1997. Complete reductive dechlorination of 1,2-dichloropropane by anaerobic bacteria. Applied and Environmental Microbiology, 63, 2870–2875. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1389209/ PMC1389209]</ref><ref name= "Samin2012" />and multiple species within the genus [[wikipedia:: Dehalogenimonas | ''Dehalogenimonas'']] capable of anaerobic reductive dechlorination of TCP have been isolated from contaminated groundwater<ref>Moe, W.M., Yan, J., Nobre, M.F., da Costa, M.S., Rainey, F.A., 2009. Dehalogenimonas lykanthroporepellens gen. nov., sp. nov., a reductively dehalogenating bacterium isolated from chlorinated solvent-contaminated groundwater. International Journal of Systematic and Evolutionary Microbiology, 59(11), 2692-2697. [https://doi.org/10.1099/ijs.0.011502-0 doi:10.1099/ijs.0.011502-0]</ref><ref>Yan, J., Rash, B.A., Rainey, F.A., Moe, W.M., 2009. Isolation of novel bacteria within the Chloroflexi capable of reductive dechlorination of 1, 2, 3‐trichloropropane. Environmental Microbiology, 11(4), 833-843. [https://doi.org/10.1111/j.1462-2920.2008.01804.x doi:10.1111/j.1462-2920.2008.01804.x]</ref><ref>Bowman, K.S., M.F. Nobre, M.S. da Costa, F.A. Rainy, Moe, W.M., 2013. Dehalogenimonas alkenigignens sp. nov., a chlorinated-alkane-dehalogenating bacterium isolated from groundwater. International Journal of Systematic and Evolutionary Microbiology, 63, 1492-1498. [https://www.ncbi.nlm.nih.gov/pubmed/22888191 doi: 10.1099/ijs.0.045054-0]</ref>. A bioaugmentation culture containing ''Dehalogenimonas'' (KB-1 Plus, SiREM) is commercially available and has been implemented for remediation of TCP-contaminated groundwater<ref>Schmitt, M., Suchomel, E., 2016. 1,2,3-TCP Remediation in Groundwater: Biological Reduction and Chemical Reduction using ZVZ. Emerging Contaminants Summit. Westminster, CO.</ref>.
| + | ==Mobile Porosity== |
| | + | More recently, data from multiple short-term tracer tests conducted to design in situ remediation systems, have been analyzed to better understand contaminant migration in groundwater<ref name= "Payne2008">Payne, F.C., Quinnan, J.A. and Potter, S.T., 2008. Remediation hydraulics. CRC Press. ISBN 978-1-4200-0684-1</ref>. In these tests, the dissolved solutes were observed to migrate more rapidly through the aquifer than could be explained with typically reported values of n<sub>e</sub>. The interpretation is that the heterogeneity of unconsolidated formations results in a relatively small area of an aquifer cross section carrying most of the water, and so solutes migrate more rapidly than expected. Based on these results, the recommendation is that a quantity called “mobile porosity” should be used in place of n<sub>e</sub> in equation 2 for calculating solute transport velocities. Based on 15 different tracer tests, typical values of mobile porosity range from 0.02 to 0.10 (Table 2). |
| | | | |
| − | ==Summary==
| + | [[File:Newell-Article 1-Table2r2.jpg|500px|center|thumbnail|Table 2. Mobile porosity estimates from tracer tests<ref name= "Payne2008"/>.]] |
| − | The relatively high toxicity of TCP has led to the development of low health-based drinking water concentration goals. TCP is present in groundwater and in public water systems at concentrations that exceed these health-based goals. While the EPA has not established a MCL for TCP, a handful of states have either established MCLs or are in the process of doing so. Because TCP is persistent in groundwater and it is resistant to typical remediation methods (or costly to treat), specialized strategies may be needed to meet drinking-water-based treatment goals. Research into TCP treatment is ongoing.
| |
| | | | |
| | ==References== | | ==References== |
| Line 51: |
Line 59: |
| | | | |
| | ==See Also== | | ==See Also== |
| − | *[https://www.atsdr.cdc.gov/ToxProfiles/TP.asp?id=912&tid=186 ATSDR Toxicological Profile] | + | *[http://iwmi.dhigroup.com/solute_transport/advection.html International Water Management Institute Animations] |
| − | *[https://www.epa.gov/sites/production/files/2014-03/documents/ffrrofactsheet_contaminant_tcp_january2014_final.pdf EPA Technical Fact Sheet] | + | *[http://www2.nau.edu/~doetqp-p/courses/env303a/lec32/lec32.htm NAU Lecture Notes on Advective Transport] |
| − | *[http://www.waterboards.ca.gov/gama/docs/coc_tcp123.pdf Cal/EPA State Water Resources Control Board Groundwater Information Sheet] | + | *[https://www.youtube.com/watch?v=00btLB6u6DY MIT Open CourseWare Solute Transport: Advection with Dispersion Video] |
| − | *[http://www.waterboards.ca.gov/drinking_water/certlic/drinkingwater/documents/123-tcp/123tcp_factsheet.pdf California Water Boards Fact Sheet] | + | *[https://www.youtube.com/watch?v=AtJyKiA1vcY Physical Groundwater Model Video] |
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Emerging-Issues/ER-1457 Prospects for Remediation of Advection and Groundwater Flow by Natural and Engineered Abiotic Degradation Reactions] | + | *[https://www.coursera.org/learn/natural-attenuation-of-groundwater-contaminants/lecture/UzS8q/groundwater-flow-review Online Lecture Course - Groundwater Flow] |
Advection is the movement of groundwater through the subsurface due to pressure and gravitational energy. The combined processes of advection, dispersion, diffusion, sorption, and degradation control how long a contaminant plume will grow, remain stable, or shrink, as well as how easy or difficult it will be to remediate. The combined effects of these processes are represented in solute transport models with the advection-dispersion-reaction equation.
Related Article(s):
CONTRIBUTOR(S): Dr. Charles Newell, P.E.
Key Resource(s):
Groundwater Flow
Groundwater will flow from areas of high hydraulic head (pressure and gravitational energy) to areas of lower hydraulic head (Fig. 1). The slope of the change in hydraulic head is known as the hydraulic gradient. If groundwater is flowing and contains dissolved contaminants it can transport the contaminants from areas with high hydraulic head to lower hydraulic head (or “downgradient”).
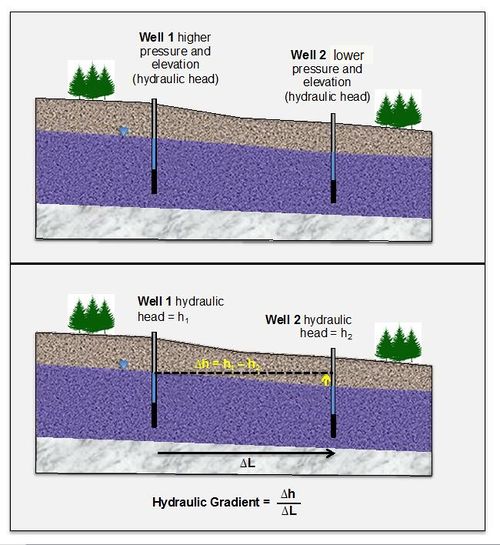
Figure 1. Hydraulic gradient (typically described in units of m/m or ft/ft) is the difference in hydraulic head from Point A to Point B (ΔH) divided by the distance between them (ΔL). In unconfined aquifers, the hydraulic gradient can also be described as the slope of the water table (Adapted from course notes developed by Dr. R.J. Mitchell, Western Washington University).
Darcy's Law
In unconsolidated geologic settings (gravel, sand, silt, and clay) and highly fractured systems, the rate of groundwater movement can be expressed using Darcy’s Law. This law is a fundamental mathematical relationship in the groundwater field and can be expressed this way:
- Where:
- Q = Flow per area (Volume groundwater flow per area per time, such as m3/yr)
- A = Cross sectional area perpendicular to groundwater flow (length2, such as m2)
- VD = “Darcy Velocity”; another way to describe groundwater flow as the flow per unit area (units of length per time, such as ft/yr)
- K = Hydraulic Conductivity (sometimes called “permeability”) (length per time)
- ΔH = Difference in hydraulic head between two lateral points (length)
- ΔL = Length between two lateral points (length)
Hydraulic conductivity (Table 1 and Fig. 2) is a measure of how easy groundwater flows through a porous medium, or alternatively, how much energy it takes to force water through a porous medium. For example, fine sand (sand with small grains) means smaller pores and more frictional resistance and therefore lower hydraulic conductivity (Fig. 2) compared to coarse sand (sand with large grains), which has less resistance to flow.
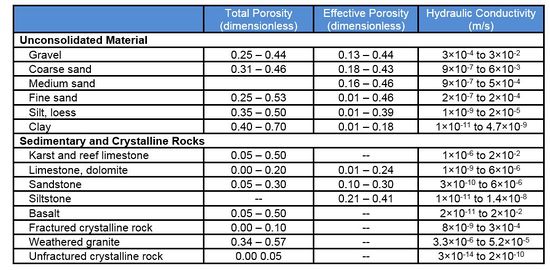
Table 1. Representative values of total porosity (n), effective porosity (n
e), and hydraulic conductivity (K) for different aquifer materials
[1][2][3].
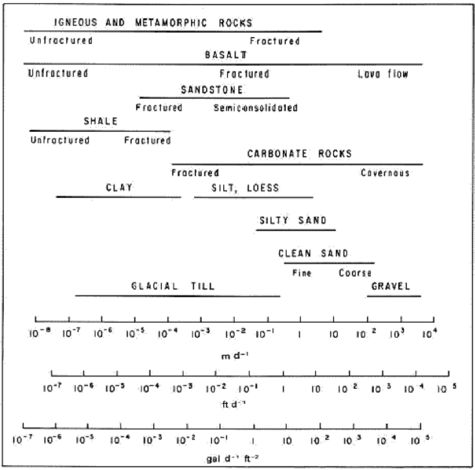
Figure 2. Hydraulic conductivity of selected rocks
[4].
Darcy’s Law was first described by Henry Darcy (1856)[5] in a report regarding a water supply system he designed for the city of Dijon, France. He ran experiments and concluded that the amount of water flowing through a closed tube of sand (dark grey box in Figure 3) depends on (a) the change in the hydraulic head between the inlet and outlet of the tube, and (b) the hydraulic conductivity of the sand in the tube. Groundwater flows rapidly in the case of higher pressure (ΔH) and permeable materials such as gravel or coarse sand, but flows slowly when the pressure is lower and low-permeability material such as fine sand or silt.

Figure 3. Conceptual explanation of Darcy’s Law based on Darcy’s experiment (Adapted from course notes developed by Dr. R.J. Mitchell, Western Washington University).
Since Darcy’s time, Darcy’s Law has been adapted to calculate the actual velocity that the groundwater is moving in units such as meters traveled per year. This quantity is called “interstitial velocity” or “seepage velocity” and is calculated by dividing the Darcy Velocity (flow per unit area) by the actual open pore area where groundwater is flowing, the “effective porosity” (Table 1):

- Where:
- VS = “interstitial velocity” or “seepage velocity” (units of length per time, such as m/sec)
- VD = “Darcy Velocity”; another way to describe groundwater flow as the flow per unit area (units of length per time)
- ne = Effective porosity (unitless)
Effective porosity is smaller than total porosity. The difference is that total porosity includes some dead-end pores that do not support groundwater. Typically values for total and effective porosity are shown in Table 1.
Darcy Velocity and Seepage Velocity
In groundwater calculations, Darcy Velocity and Seepage Velocity are two different things used for different purposes. For any calculation where the actual flow rate in units of volume per time (such as liters per day or gallons per minute) is involved, then the original Darcy Equation should be used (calculate VD; Equation 1) without using effective porosity. When calculating solute travel time, then the seepage velocity calculation (VS; Equation 2) must be used and an estimate of effective porosity is required. Figure 4 illustrates the differences between Darcy Velocity and Seepage Velocity.
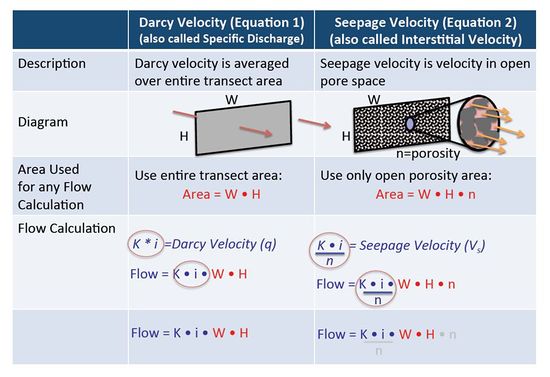
Figure 4. Difference between Darcy Velocity (also called Specific Discharge) and Seepage Velocity (also called Interstitial Velocity).
Mobile Porosity
More recently, data from multiple short-term tracer tests conducted to design in situ remediation systems, have been analyzed to better understand contaminant migration in groundwater[6]. In these tests, the dissolved solutes were observed to migrate more rapidly through the aquifer than could be explained with typically reported values of ne. The interpretation is that the heterogeneity of unconsolidated formations results in a relatively small area of an aquifer cross section carrying most of the water, and so solutes migrate more rapidly than expected. Based on these results, the recommendation is that a quantity called “mobile porosity” should be used in place of ne in equation 2 for calculating solute transport velocities. Based on 15 different tracer tests, typical values of mobile porosity range from 0.02 to 0.10 (Table 2).
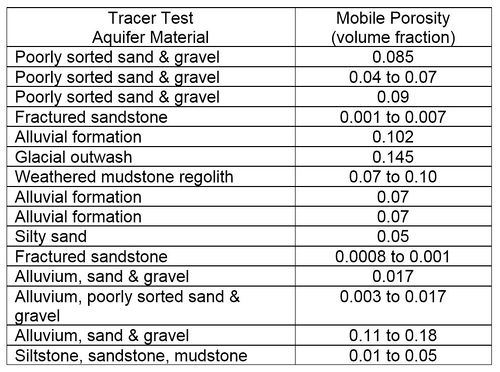
Table 2. Mobile porosity estimates from tracer tests
[6].
References
- ^ 1.0 1.1 Domenico, P.A. and Schwartz, F.W., 1998. Physical and chemical hydrogeology. John Wiley & Sons, 2nd Ed., 528 pgs. ISBN 978-0-471-59762-9.
- ^ McWhorter, D.B. and Sunada, D.K., 1977. Ground-water hydrology and hydraulics. Water Resources Publication, LLC. 304 pgs. ISBN 978-0-918334-18-3
- ^ Freeze, R.A. and Cherry, J.A., 1979. Groundwater. 604 pgs. ISBN 978-0133653120
- ^ Heath, R.C., 1983. Basic ground-water hydrology, U.S. Geological Survey Water-Supply Paper 2220, 86 pgs. Report pdf
- ^ Darcy, H., 1856. Les Fontaines Publiques de la Ville de Dijon, Dalmont, Paris. doi: 10.1029/2001WR000727
- ^ 6.0 6.1 Payne, F.C., Quinnan, J.A. and Potter, S.T., 2008. Remediation hydraulics. CRC Press. ISBN 978-1-4200-0684-1
See Also






