Difference between revisions of "Bioremediation - Anaerobic"
m |
(Tag: Visual edit) |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
Bioremediation is the process by which contaminants in soil and/or groundwater are treated biologically, primarily by microorganisms or biomolecules generated by the cells. Bioremediation processes can take place under oxic (with oxygen) or anoxic (without oxygen) conditions. This article focuses on enhanced in situ bioremediation (EISB) for the anaerobic biodegradation of organic contaminants, particularly [[Chlorinated Solvents | chlorinated solvents]], in soil and groundwater. However, much of the information provided is applicable to other contaminant types. EISB is frequently selected as a remedial technology as it can provide complete degradation of contaminants utilizing natural microbial processes, is able to be implemented in a variety of site conditions, and is relatively low cost compared to more active engineered remedial systems. | Bioremediation is the process by which contaminants in soil and/or groundwater are treated biologically, primarily by microorganisms or biomolecules generated by the cells. Bioremediation processes can take place under oxic (with oxygen) or anoxic (without oxygen) conditions. This article focuses on enhanced in situ bioremediation (EISB) for the anaerobic biodegradation of organic contaminants, particularly [[Chlorinated Solvents | chlorinated solvents]], in soil and groundwater. However, much of the information provided is applicable to other contaminant types. EISB is frequently selected as a remedial technology as it can provide complete degradation of contaminants utilizing natural microbial processes, is able to be implemented in a variety of site conditions, and is relatively low cost compared to more active engineered remedial systems. | ||
<div style="float:right;margin:0 0 2em 2em;">__TOC__</div> | <div style="float:right;margin:0 0 2em 2em;">__TOC__</div> | ||
| + | '''Related Article(s):''' | ||
| − | |||
| − | |||
*[[Bioremediation - Anaerobic Design Considerations]] | *[[Bioremediation - Anaerobic Design Considerations]] | ||
| + | *[[Bioremediation - Anaerobic Secondary Water Quality Impacts]] | ||
| + | *[[Design Tool - Base Addition for ERD]] | ||
*[[Low pH Inhibition of Reductive Dechlorination]] | *[[Low pH Inhibition of Reductive Dechlorination]] | ||
| − | |||
| − | |||
| − | ''' | + | '''Contributor(s):''' [[Michaye McMaster, M.Sc.|Michaye McMaster]] and [[Leah MacKinnon, M.A.Sc., P. Eng.|Leah MacKinnon, M.A.Sc., P.E.]] |
'''Key Resource(s)''': | '''Key Resource(s)''': | ||
| − | |||
| − | |||
| + | *[//www.enviro.wiki/images/4/44/USEPA-2013-introductiontoinsitubioremediationofgroundwater.pdf Introduction to In Situ Bioremediation of Groundwater. EPA 542-R-13-018]<ref name="USEPA2013Intro">USEPA, 2013. Introduction to In Situ Bioremediation of Groundwater. EPA 542-R-13-018. [//www.enviro.wiki/images/4/44/USEPA-2013-introductiontoinsitubioremediationofgroundwater.pdf Report pdf]</ref> | ||
| + | *[http://www.navfac.navy.mil/content/dam/navfac/Specialty%20Centers/Engineering%20and%20Expeditionary%20Warfare%20Center/Environmental/Restoration/er_pdfs/d/navfacexwc-ev-tm-1501-erd-design-201503f.pdf Design Considerations for Enhanced Reductive Dechlorination. TM-NAVFAC-EXWC-EV-1501]<ref name="NAVFAC2015D">NAVFAC, 2015. Design considerations for Enhanced Reductive Dechlorination. TM-NAVFAC-EXWC-EV-1501. [http://www.navfac.navy.mil/content/dam/navfac/Specialty%20Centers/Engineering%20and%20Expeditionary%20Warfare%20Center/Environmental/Restoration/er_pdfs/d/navfacexwc-ev-tm-1501-erd-design-201503f.pdf Report pdf]</ref> | ||
==Introduction== | ==Introduction== | ||
| Line 22: | Line 21: | ||
==Degradation Processes== | ==Degradation Processes== | ||
| − | Under anaerobic conditions, organic contaminants can serve as the electron acceptors or electron donors during biodegradation processes<ref name= "USEPA2013Intro"/>; we refer to the former as “anaerobic reductive bioremediation” and the latter as “anaerobic oxidative bioremediation”. | + | Under anaerobic conditions, organic contaminants can serve as the electron acceptors or electron donors during biodegradation processes<ref name="USEPA2013Intro" />; we refer to the former as “anaerobic reductive bioremediation” and the latter as “anaerobic oxidative bioremediation”. |
| − | *Anaerobic reductive bioremediation relies on the presence of biologically available organic carbon, which may be naturally present or added to stimulate biological activity. Organic bioremediation amendments, referred to as organic substrates or electron donors, generate and sustain anoxic conditions by consuming oxygen via aerobic respiration, as well as other electron acceptors, during its biodegradation. For example, [[Chlorinated Solvents | chlorinated solvents]] such as [[wikipedia:Trichloroethylene | trichloroethene (TCE)]] serve as electron acceptors and undergo [[Biodegradation - Reductive Processes | reductive dechlorination]] under anaerobic conditions in the presence of an electron donor. This microbial-mediated process can result in the complete degradation of many specific chlorinated solvents to innocuous end products. | + | |
| − | *Anaerobic oxidative bioremediation relies on other electron acceptors such as nitrate or sulfate for direct microbial metabolic oxidation of a contaminant serving as the electron donor. This approach may be applied for the treatment of non-chlorinated hydrocarbon compounds where oxygen has already been depleted. | + | *Anaerobic reductive bioremediation relies on the presence of biologically available organic carbon, which may be naturally present or added to stimulate biological activity. Organic bioremediation amendments, referred to as organic substrates or electron donors, generate and sustain anoxic conditions by consuming oxygen via aerobic respiration, as well as other electron acceptors, during its biodegradation. For example, [[Chlorinated Solvents | chlorinated solvents]] such as [[wikipedia:Trichloroethylene | trichloroethene (TCE)]] serve as electron acceptors and undergo [[Biodegradation - Reductive Processes | reductive dechlorination]] under anaerobic conditions in the presence of an electron donor. This microbial-mediated process can result in the complete degradation of many specific chlorinated solvents to innocuous end products. |
| + | *Anaerobic oxidative bioremediation relies on other electron acceptors such as nitrate or sulfate for direct microbial metabolic oxidation of a contaminant serving as the electron donor. This approach may be applied for the treatment of non-chlorinated hydrocarbon compounds where oxygen has already been depleted. | ||
In contrast, [[Biodegradation - Cometabolic | cometabolism]] occurs when microorganisms do not utilize the organic contaminant as an energy source, but the contaminant is fortuitously degraded by enzymes or co-factors produced during the metabolism of another compound. | In contrast, [[Biodegradation - Cometabolic | cometabolism]] occurs when microorganisms do not utilize the organic contaminant as an energy source, but the contaminant is fortuitously degraded by enzymes or co-factors produced during the metabolism of another compound. | ||
| Line 34: | Line 34: | ||
|+Table 1. Biodegradation processes for perchlorate and common organic contaminants. | |+Table 1. Biodegradation processes for perchlorate and common organic contaminants. | ||
|- | |- | ||
| − | ! Contaminant!! Aerobic Oxidation!! Aerobic Cometabolism!! Anaerobic Oxidation !! Anaerobic Reduction!! Cometabolic Anaerobic Reduction | + | !Contaminant!!Aerobic Oxidation!!Aerobic Cometabolism!!Anaerobic Oxidation!!Anaerobic Reduction!!Cometabolic Anaerobic Reduction |
|- | |- | ||
| − | | Perchlorate<ref name= "USEPA2013Intro"/><ref>Stroo, H.F., Loehr, R.C., Ward, C.H. eds., 2008. In situ bioremediation of perchlorate in groundwater. Springer Science & Business Media. [https://doi.org/10.1007/978-0-387-84921-8_1 doi: 10.1007/978-0-387-84921-8_10]</ref>||[[File:Circle with diagnal line.PNG|15px]]||[[File:Circle with diagnal line.PNG|15px]]||[[File:Circle with diagnal line.PNG|15px]] | + | |Perchlorate<ref name="USEPA2013Intro" /><ref>Stroo, H.F., Loehr, R.C., Ward, C.H. eds., 2008. In situ bioremediation of perchlorate in groundwater. Springer Science & Business Media. [https://doi.org/10.1007/978-0-387-84921-8_1 doi: 10.1007/978-0-387-84921-8_10]</ref>||[[File:Circle with diagnal line.PNG|15px]]||[[File:Circle with diagnal line.PNG|15px]]||[[File:Circle with diagnal line.PNG|15px]] |
| − | ||[[File:Circle black fill.PNG|15px]]|| [[File:Circle with diagnal line.PNG|15px]] | + | ||[[File:Circle black fill.PNG|15px]]||[[File:Circle with diagnal line.PNG|15px]] |
|- | |- | ||
| − | | TNT/RDX/HMX<ref>Spain, J.C., Hughes, J.B. and Knackmuss, H.J. eds., 2000. Biodegradation of nitroaromatic compounds and explosives. CRC Press</ref><ref name= "Kalderis2011">Kalderis, D., Juhasz, A.L., Boopathy, R. and Comfort, S., 2011. Soils contaminated with explosives: Environmental fate and evaluation of state-of-the-art remediation processes (IUPAC Technical Report). Pure and Applied Chemistry, 83(7), 1407-1484. [https://doi.org/10.1351/pac-rep-10-01-05 doi:10.1351/pac-rep-10-01-05]</ref><ref>Battelle, 2015. Attenuation Pathways for Munitions Constituents in Soils and Groundwater, NAVFAC Technical Report - TR-NAVFAC-EXWC-EV-1503 [ | + | |TNT/RDX/HMX<ref>Spain, J.C., Hughes, J.B. and Knackmuss, H.J. eds., 2000. Biodegradation of nitroaromatic compounds and explosives. CRC Press</ref><ref name="Kalderis2011">Kalderis, D., Juhasz, A.L., Boopathy, R. and Comfort, S., 2011. Soils contaminated with explosives: Environmental fate and evaluation of state-of-the-art remediation processes (IUPAC Technical Report). Pure and Applied Chemistry, 83(7), 1407-1484. [https://doi.org/10.1351/pac-rep-10-01-05 doi:10.1351/pac-rep-10-01-05]</ref><ref>Battelle, 2015. Attenuation Pathways for Munitions Constituents in Soils and Groundwater, NAVFAC Technical Report - TR-NAVFAC-EXWC-EV-1503 [//www.enviro.wiki/images/5/55/NAVFAC-2015-AttenuationPathways.pdf Report pdf]</ref>||[[File:Circle black fill.PNG|15px]][[File:Circle dark round-middle white.PNG|15px]]||[[File:Circle black fill.PNG|15px]][[File:Circle dark round-middle white.PNG|15px]]||[[File:Circle with diagnal line.PNG|15px]]||[[File:Circle black fill.PNG|15px]]||[[File:Circle black fill.PNG|15px]] |
|- | |- | ||
| − | | 1,4-dioxane<ref>Mahendra, S. and Alvarez-Cohen, L., 2006. Kinetics of 1, 4-dioxane biodegradation by monooxygenase-expressing bacteria. Environmental Science & Technology, 40(17), 5435-5442. [https://doi.org/10.1021/es060714v doi:10.1021/es060714v]</ref><ref>Steffan, R.J., McClay, K.R., Masuda, H. and Zylstra, G.J., 2007. ER-1422: Biodegradation of 1, 4-Dioxane No. CU-1422. Shaw Environmental Inc Lawrenceville NJ. [https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Emerging-Issues/ER-1422/ER-1422/(language)/eng-US ER-1422]</ref><ref>Adamson, D.T., Anderson, R.H., Mahendra, S. and Newell, C.J., 2015. Evidence of 1, 4-dioxane attenuation at groundwater sites contaminated with chlorinated solvents and 1, 4-dioxane. Environmental Science & Technology, 49(11), 6510-6518. [https://doi.org/10.1021/acs.est.5b00964 doi:10.1021/acs.est.5b00964]</ref>||[[File:Circle Open.PNG|15px]]|| [[File:Circle black fill.PNG|15px]]||[[ File:Circle with diagnal line.PNG|15px]]|| [[File:Circle with diagnal line.PNG|15px]]|| [[File:Circle with diagnal line.PNG|15px]] | + | |1,4-dioxane<ref>Mahendra, S. and Alvarez-Cohen, L., 2006. Kinetics of 1, 4-dioxane biodegradation by monooxygenase-expressing bacteria. Environmental Science & Technology, 40(17), 5435-5442. [https://doi.org/10.1021/es060714v doi:10.1021/es060714v]</ref><ref>Steffan, R.J., McClay, K.R., Masuda, H. and Zylstra, G.J., 2007. ER-1422: Biodegradation of 1, 4-Dioxane No. CU-1422. Shaw Environmental Inc Lawrenceville NJ. [https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Emerging-Issues/ER-1422/ER-1422/(language)/eng-US ER-1422]</ref><ref>Adamson, D.T., Anderson, R.H., Mahendra, S. and Newell, C.J., 2015. Evidence of 1, 4-dioxane attenuation at groundwater sites contaminated with chlorinated solvents and 1, 4-dioxane. Environmental Science & Technology, 49(11), 6510-6518. [https://doi.org/10.1021/acs.est.5b00964 doi:10.1021/acs.est.5b00964]</ref>||[[File:Circle Open.PNG|15px]]||[[File:Circle black fill.PNG|15px]]||[[ File:Circle with diagnal line.PNG|15px]]||[[File:Circle with diagnal line.PNG|15px]]||[[File:Circle with diagnal line.PNG|15px]] |
|- | |- | ||
| − | | Chloroethenes<ref name= "Wiedemeir1999">Wiedemeier, T.H., Newell, C.J., Rifai, H.S., and Wilson, J.T., 1999. Natural attenuation of fuels and chlorinated solvents in the subsurface. John Wiley & Sons. [https://doi.org/10.1002/9780470172964 doi:1 0.1002/9780470172964]</ref><ref name="Lawrence2006">Lawrence, S.J., 2006. Description, Properties, and Degradation of Selected Volatile Organic Compounds Detected in Ground Water--A Review of Selected Literature No. 2006-1338. [ | + | |Chloroethenes<ref name="Wiedemeir1999">Wiedemeier, T.H., Newell, C.J., Rifai, H.S., and Wilson, J.T., 1999. Natural attenuation of fuels and chlorinated solvents in the subsurface. John Wiley & Sons. [https://doi.org/10.1002/9780470172964 doi:1 0.1002/9780470172964]</ref><ref name="Lawrence2006">Lawrence, S.J., 2006. Description, Properties, and Degradation of Selected Volatile Organic Compounds Detected in Ground Water--A Review of Selected Literature No. 2006-1338. [//www.enviro.wiki/images/5/5f/Lawrence-2006-Description_properties_degradation_of_VOCs.pdf Report pdf]</ref>||[[File:Circle black fill.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]]||[[File:Circle black fill.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]]||[[File:Circle black fill.PNG|15px]][[File:Circle dark round-middle white.PNG|15px]]||[[File:Circle black fill.PNG|15px]]||[[File:Circle black fill.PNG|15px]] |
|- | |- | ||
| − | | Chloroethanes<ref name= "Wiedemeir1999"/><ref name="Lawrence2006"/>|| [[File:Circle black fill.PNG|15px]][[File:Circle dark round-middle white.PNG|15px]]|| [[File:Circle black fill.PNG|15px]][[File:Circle dark round-middle white.PNG|15px]]|| [[File:Circle with diagnal line.PNG|15px]]|| [[File:Circle black fill.PNG|15px]][[File:Circle dark round-middle white.PNG|15px]]|| [[File:Circle black fill.PNG|15px]][[File:Circle dark round-middle white.PNG|15px]] | + | |Chloroethanes<ref name="Wiedemeir1999" /><ref name="Lawrence2006" />||[[File:Circle black fill.PNG|15px]][[File:Circle dark round-middle white.PNG|15px]]||[[File:Circle black fill.PNG|15px]][[File:Circle dark round-middle white.PNG|15px]]||[[File:Circle with diagnal line.PNG|15px]]||[[File:Circle black fill.PNG|15px]][[File:Circle dark round-middle white.PNG|15px]]||[[File:Circle black fill.PNG|15px]][[File:Circle dark round-middle white.PNG|15px]] |
|- | |- | ||
| − | | Chloromethanes<ref name= "Wiedemeir1999"/><ref name="Lawrence2006"/><ref name= "Maness2012">Maness, A.D., Bowman, K.S., Yan, J., Rainey, F.A. and Moe, W.M., 2012. Dehalogenimonas spp. can reductively dehalogenate high concentrations of 1, 2-dichloroethane, 1, 2-dichloropropane, and 1, 1, 2-trichloroethane. AMB Express, 2(1), 54. [https://doi.org/10.1186/2191-0855-2-54 doi:10.1186/2191-0855-2-54]</ref>|| [[File:Circle black fill.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]] || [[File:Circle black fill.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]]|| [[File:Circle black fill.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]]|| [[File:Circle black fill.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]]|| [[File:Circle black fill.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]] | + | |Chloromethanes<ref name="Wiedemeir1999" /><ref name="Lawrence2006" /><ref name="Maness2012">Maness, A.D., Bowman, K.S., Yan, J., Rainey, F.A. and Moe, W.M., 2012. Dehalogenimonas spp. can reductively dehalogenate high concentrations of 1, 2-dichloroethane, 1, 2-dichloropropane, and 1, 1, 2-trichloroethane. AMB Express, 2(1), 54. [https://doi.org/10.1186/2191-0855-2-54 doi:10.1186/2191-0855-2-54]</ref>||[[File:Circle black fill.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]]||[[File:Circle black fill.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]]||[[File:Circle black fill.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]]||[[File:Circle black fill.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]]||[[File:Circle black fill.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]] |
|- | |- | ||
| − | | Chlorobenzenes<ref name= "Spain1995">Spain, J.C. ed., 1995. Biodegradation of nitroaromatic compounds. Environmental Science Research, 49. [https://doi.org/10.1007/978-1-4757-9447-2 doi 10.1007/978-1-4757-9447-2]</ref><ref name= "Field2008">Field, J.A. and Sierra-Alvarez, R., 2008. Microbial degradation of chlorinated benzenes. Biodegradation, 19(4), 463-480. [https://doi.org/10.1007/s10532-007-9155-1 doi:10.1007/s10532-007-9155-1]</ref><ref>Adrian, L. and Görisch, H., 2002. Microbial transformation of chlorinated benzenes under anaerobic conditions. Research in Microbiology, 153(3), 131-137. [https://doi.org/10.1016/s0923-2508(02)01298-6 doi:10.1016/s0923-2508(02)01298-6]</ref>|| [[File:Circle black fill.PNG|15px]] || [[File:Circle with diagnal line.PNG|15px]]|| [[File:Circle with diagnal line.PNG|15px]]|| [[File:Circle black fill.PNG|15px]]|| [[File:Circle Open.PNG|15px]] | + | |Chlorobenzenes<ref name="Spain1995">Spain, J.C. ed., 1995. Biodegradation of nitroaromatic compounds. Environmental Science Research, 49. [https://doi.org/10.1007/978-1-4757-9447-2 doi 10.1007/978-1-4757-9447-2]</ref><ref name="Field2008">Field, J.A. and Sierra-Alvarez, R., 2008. Microbial degradation of chlorinated benzenes. Biodegradation, 19(4), 463-480. [https://doi.org/10.1007/s10532-007-9155-1 doi:10.1007/s10532-007-9155-1]</ref><ref>Adrian, L. and Görisch, H., 2002. Microbial transformation of chlorinated benzenes under anaerobic conditions. Research in Microbiology, 153(3), 131-137. [https://doi.org/10.1016/s0923-2508(02)01298-6 doi:10.1016/s0923-2508(02)01298-6]</ref>||[[File:Circle black fill.PNG|15px]]||[[File:Circle with diagnal line.PNG|15px]]||[[File:Circle with diagnal line.PNG|15px]]||[[File:Circle black fill.PNG|15px]]||[[File:Circle Open.PNG|15px]] |
|- | |- | ||
| − | | Nitrobenzenes<ref name= "Kalderis2011"/><ref name= "Spain1995"/>|| [[File:Circle black fill.PNG|15px]]|| [[File:Circle Open.PNG|15px]][[File:Circle dark round-middle white.PNG|15px]]|| [[File:Circle with diagnal line.PNG|15px]]|| [[File:Circle black fill.PNG|15px]]|| [[File:Circle with diagnal line.PNG|15px]] | + | |Nitrobenzenes<ref name="Kalderis2011" /><ref name="Spain1995" />||[[File:Circle black fill.PNG|15px]]||[[File:Circle Open.PNG|15px]][[File:Circle dark round-middle white.PNG|15px]]||[[File:Circle with diagnal line.PNG|15px]]||[[File:Circle black fill.PNG|15px]]||[[File:Circle with diagnal line.PNG|15px]] |
|- | |- | ||
| − | | BTEX<ref name= "Wiedemeir1999"/><ref name="Lawrence2006"/><ref>Prenafeta-Boldú, F.X., Vervoort, J., Grotenhuis, J.T.C. and Van Groenestijn, J.W., 2002. Substrate interactions during the biodegradation of benzene, toluene, ethylbenzene, and xylene (BTEX) hydrocarbons by the fungus Cladophialophora sp. strain T1. Applied and Environmental Microbiology, 68(6), 2660-2665. [https://doi.org/10.1128/aem.68.6.2660-2665.2002 doi: 10.1128/aem.68.6.2660-2665.2002]</ref>|| [[File:Circle black fill.PNG|15px]]|| [[File:Circle Open.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]] || [[File:Circle black fill.PNG|15px]]|| [[File:Circle with diagnal line.PNG|15px]]|| [[File:Circle with diagnal line.PNG|15px]] | + | |BTEX<ref name="Wiedemeir1999" /><ref name="Lawrence2006" /><ref>Prenafeta-Boldú, F.X., Vervoort, J., Grotenhuis, J.T.C. and Van Groenestijn, J.W., 2002. Substrate interactions during the biodegradation of benzene, toluene, ethylbenzene, and xylene (BTEX) hydrocarbons by the fungus Cladophialophora sp. strain T1. Applied and Environmental Microbiology, 68(6), 2660-2665. [https://doi.org/10.1128/aem.68.6.2660-2665.2002 doi: 10.1128/aem.68.6.2660-2665.2002]</ref>||[[File:Circle black fill.PNG|15px]]||[[File:Circle Open.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]]||[[File:Circle black fill.PNG|15px]]||[[File:Circle with diagnal line.PNG|15px]]||[[File:Circle with diagnal line.PNG|15px]] |
|- | |- | ||
| − | | MTBE and TBA<ref>Zeeb, P. and Wiedemeier, T.H., 2007. Technical protocol for evaluating the natural attenuation of MTBE. API Publication 4761. [ | + | |MTBE and TBA<ref>Zeeb, P. and Wiedemeier, T.H., 2007. Technical protocol for evaluating the natural attenuation of MTBE. API Publication 4761. [//www.enviro.wiki/images/9/9f/API_MNA_MTBE_protocol.pdf Report pdf]</ref><ref>USEPA, 2007. Monitored Natural Attenuation of Tertiary Butyl Alcohol (TBA) in Ground Water at Gasoline Spill Sites. EPA 600-R-07-100. [[Special:FilePath/Epa 600-r-07-100.pdf|Report pdf]]</ref>||[[File:Circle black fill.PNG|15px]]||[[File:Circle black fill.PNG|15px]]||[[File:Circle black fill.PNG|15px]]||[[File:Circle black fill.PNG|15px]]||[[File:Circle Open.PNG|15px]] |
|- | |- | ||
| − | | Chloropropanes<ref name= "Maness2012"/><ref>Schlötelburg, C., Von Wintzingerode, F., Hauck, R., Hegemann, W. and Göbel, U.B., 2000. Bacteria of an anaerobic 1, 2-dichloropropane-dechlorinating mixed culture are phylogenetically related to those of other anaerobic dechlorinating consortia. International Journal of Systematic and Evolutionary Microbiology, 50(4), 1505-1511. [https://doi.org/10.1099/00207713-50-4-1505 doi:10.1099/00207713-50-4-1505]</ref> || [[File:Circle black fill.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]]|| [[File:Circle black fill.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]] || [[File:Circle with diagnal line.PNG|15px]]|| [[File:Circle black fill.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]]|| [[File:Circle black fill.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]] | + | |Chloropropanes<ref name="Maness2012" /><ref>Schlötelburg, C., Von Wintzingerode, F., Hauck, R., Hegemann, W. and Göbel, U.B., 2000. Bacteria of an anaerobic 1, 2-dichloropropane-dechlorinating mixed culture are phylogenetically related to those of other anaerobic dechlorinating consortia. International Journal of Systematic and Evolutionary Microbiology, 50(4), 1505-1511. [https://doi.org/10.1099/00207713-50-4-1505 doi:10.1099/00207713-50-4-1505]</ref>||[[File:Circle black fill.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]]||[[File:Circle black fill.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]]||[[File:Circle with diagnal line.PNG|15px]]||[[File:Circle black fill.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]]||[[File:Circle black fill.PNG|15px]] [[File:Circle dark round-middle white.PNG|15px]] |
|- | |- | ||
| − | | colspan="12" style="color:black;text-align:left;"|Notes: [[File:Circle black fill.PNG|15px]] = Well documented, process is confirmed to occur; [[File:Circle Open.PNG|15px]] = Some amount of documentation; [[File:Circle dark round-middle white.PNG|15px]] = Only select compounds may undergo these processes; [[File:Circle with diagnal line.PNG|15px]] Not routinely documented. | + | | colspan="12" style="color:black;text-align:left;" |Notes: [[File:Circle black fill.PNG|15px]] = Well documented, process is confirmed to occur; [[File:Circle Open.PNG|15px]] = Some amount of documentation; [[File:Circle dark round-middle white.PNG|15px]] = Only select compounds may undergo these processes; [[File:Circle with diagnal line.PNG|15px]] Not routinely documented. |
|- | |- | ||
|} | |} | ||
| Line 67: | Line 67: | ||
|+Table 2. Microbial-based immobilization mechanisms for common metals and metalloids in groundwater. | |+Table 2. Microbial-based immobilization mechanisms for common metals and metalloids in groundwater. | ||
|- | |- | ||
| − | ! Metal/Metalloid!! Oxidation States<sup>a</sup> !! Aerobic oxidation!! Anaerobic reduction!! ISP via SRB<sup>b</sup> | + | !Metal/Metalloid!!Oxidation States<sup>a</sup>!!Aerobic oxidation!!Anaerobic reduction!!ISP via SRB<sup>b</sup> |
|- | |- | ||
| − | | colspan="12" style="color:black;text-align:center;"| Oxyanions | + | | colspan="12" style="color:black;text-align:center;" |Oxyanions |
|- | |- | ||
| − | | Arsenic<ref>Katsoyiannis, I.A. and Zouboulis, A.I., 2004. Application of biological processes for the removal of arsenic from groundwaters. Water Research, 38(1), 17-26. [https://doi.org/10.1016/j.watres.2003.09.011 doi 10.1016/j.watres.2003.09.011]</ref><ref>Keimowitz, A.R., Mailloux, B.J., Cole, P., Stute, M., Simpson, H.J. and Chillrud, S.N., 2007. Laboratory investigations of enhanced sulfate reduction as a groundwater arsenic remediation strategy. Environmental Science & Technology, 41(19), 6718-6724. [https://doi.org/10.1021/es061957q doi 10.1021/es061957q]</ref><ref>Onstott, T.C., Chan, E., Polizzotto, M.L., Lanzon, J. and DeFlaun, M.F., 2011. Precipitation of arsenic under sulfate reducing conditions and subsequent leaching under aerobic conditions. Applied Geochemistry, 26(3), 269-285. [https://doi.org/10.1016/j.apgeochem.2010.11.027 doi 10.1016/j.apgeochem.2010.11.027]</ref>|| As ('''III, V''')|| [[File:Circle black fill.PNG|15px]]<sup>c</sup> || [[File:Circle with diagnal line.PNG|15px]]|| [[File:Circle black fill.PNG|15px]] | + | |Arsenic<ref>Katsoyiannis, I.A. and Zouboulis, A.I., 2004. Application of biological processes for the removal of arsenic from groundwaters. Water Research, 38(1), 17-26. [https://doi.org/10.1016/j.watres.2003.09.011 doi 10.1016/j.watres.2003.09.011]</ref><ref>Keimowitz, A.R., Mailloux, B.J., Cole, P., Stute, M., Simpson, H.J. and Chillrud, S.N., 2007. Laboratory investigations of enhanced sulfate reduction as a groundwater arsenic remediation strategy. Environmental Science & Technology, 41(19), 6718-6724. [https://doi.org/10.1021/es061957q doi 10.1021/es061957q]</ref><ref>Onstott, T.C., Chan, E., Polizzotto, M.L., Lanzon, J. and DeFlaun, M.F., 2011. Precipitation of arsenic under sulfate reducing conditions and subsequent leaching under aerobic conditions. Applied Geochemistry, 26(3), 269-285. [https://doi.org/10.1016/j.apgeochem.2010.11.027 doi 10.1016/j.apgeochem.2010.11.027]</ref>||As ('''III, V''')||[[File:Circle black fill.PNG|15px]]<sup>c</sup>||[[File:Circle with diagnal line.PNG|15px]]||[[File:Circle black fill.PNG|15px]] |
|- | |- | ||
| − | | Chromium<ref>Jeyasingh, J., Somasundaram, V., Philip, L. and Bhallamudi, S.M., 2011. Pilot scale studies on the remediation of chromium contaminated aquifer using bio-barrier and reactive zone technologies. Chemical Engineering Journal, 167(1), 206-214. [https://doi.org/10.1016/j.cej.2010.12.024 doi 10.1016/j.cej.2010.12.024]</ref><ref>Freese, K., Miller, R., J Cutright, T. and Senko, J., 2014. Review of Chromite Ore Processing Residue (COPR): Past Practices, Environmental Impact and Potential Remediation Methods. Current Environmental Engineering, 1(2), 82-90. [https://doi.org/10.2174/221271780102141117101551 doi 10.2174/221271780102141117101551]</ref>|| Cr (III, '''VI''')|| [[File:Circle with diagnal line.PNG|15px]] || [[File:Circle black fill.PNG|15px]]<sup>d</sup>|| [[File:Circle black fill.PNG|15px]] | + | |Chromium<ref>Jeyasingh, J., Somasundaram, V., Philip, L. and Bhallamudi, S.M., 2011. Pilot scale studies on the remediation of chromium contaminated aquifer using bio-barrier and reactive zone technologies. Chemical Engineering Journal, 167(1), 206-214. [https://doi.org/10.1016/j.cej.2010.12.024 doi 10.1016/j.cej.2010.12.024]</ref><ref>Freese, K., Miller, R., J Cutright, T. and Senko, J., 2014. Review of Chromite Ore Processing Residue (COPR): Past Practices, Environmental Impact and Potential Remediation Methods. Current Environmental Engineering, 1(2), 82-90. [https://doi.org/10.2174/221271780102141117101551 doi 10.2174/221271780102141117101551]</ref>||Cr (III, '''VI''')||[[File:Circle with diagnal line.PNG|15px]]||[[File:Circle black fill.PNG|15px]]<sup>d</sup>||[[File:Circle black fill.PNG|15px]] |
|- | |- | ||
| − | | Selenium<ref>Hunter, W.J. and Kuykendall, L.D., 2005. Removing selenite from groundwater with an in situ biobarrier: laboratory studies. Current Microbiology, 50(3), 145-150. [https://doi.org/10.1007/s00284-004-4418-0 doi 10.1007/s00284-004-4418-0]</ref>|| Se (-II, 0, IV, '''VI''')|| [[File:Circle with diagnal line.PNG|15px]]|| [[File:Circle black fill.PNG|15px]]<sup>e</sup>|| [[File:Circle with diagnal line.PNG|15px]] | + | |Selenium<ref>Hunter, W.J. and Kuykendall, L.D., 2005. Removing selenite from groundwater with an in situ biobarrier: laboratory studies. Current Microbiology, 50(3), 145-150. [https://doi.org/10.1007/s00284-004-4418-0 doi 10.1007/s00284-004-4418-0]</ref>||Se (-II, 0, IV, '''VI''')||[[File:Circle with diagnal line.PNG|15px]]||[[File:Circle black fill.PNG|15px]]<sup>e</sup>||[[File:Circle with diagnal line.PNG|15px]] |
|- | |- | ||
| − | | Uranium<ref>Anderson, R.T., Vrionis, H.A., Ortiz-Bernad, I., Resch, C.T., Long, P.E., Dayvault, R., Karp, K., Marutzky, S., Metzler, D.R., Peacock, A., White, D.C., , Lowe, M., Lovley, D.R., 2003. Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Applied and Environmental Microbiology, 69(10), 5884-5891. [https://doi.org/10.1128/aem.69.10.5884-5891.2003 doi 10.1128/aem.69.10.5884-5891.2003]</ref><ref>Finneran, K.T., Anderson, R.T., Nevin, K.P. and Lovley, D.R., 2002. Potential for bioremediation of uranium-contaminated aquifers with microbial U(VI) reduction. Soil and Sediment Contamination: An International Journal, 11(3), 339-357. [https://doi.org/10.1080/20025891106781 doi 10.1080/20025891106781]</ref>|| U (IV, '''VI''')|| [[File:Circle with diagnal line.PNG|15px]]|| [[File:Circle black fill.PNG|15px]]<sup>e</sup>||[[File:Circle black fill.PNG|15px]] | + | |Uranium<ref>Anderson, R.T., Vrionis, H.A., Ortiz-Bernad, I., Resch, C.T., Long, P.E., Dayvault, R., Karp, K., Marutzky, S., Metzler, D.R., Peacock, A., White, D.C., , Lowe, M., Lovley, D.R., 2003. Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Applied and Environmental Microbiology, 69(10), 5884-5891. [https://doi.org/10.1128/aem.69.10.5884-5891.2003 doi 10.1128/aem.69.10.5884-5891.2003]</ref><ref>Finneran, K.T., Anderson, R.T., Nevin, K.P. and Lovley, D.R., 2002. Potential for bioremediation of uranium-contaminated aquifers with microbial U(VI) reduction. Soil and Sediment Contamination: An International Journal, 11(3), 339-357. [https://doi.org/10.1080/20025891106781 doi 10.1080/20025891106781]</ref>||U (IV, '''VI''')||[[File:Circle with diagnal line.PNG|15px]]||[[File:Circle black fill.PNG|15px]]<sup>e</sup>||[[File:Circle black fill.PNG|15px]] |
|- | |- | ||
| − | | colspan="12" style="color:black;text-align:center;"| Metal Cations Notes: | + | | colspan="12" style="color:black;text-align:center;" |Metal Cations Notes: |
|- | |- | ||
| − | | Iron<ref name="Dvorak1992">Dvorak, D.H., Hedin, R.S., Edenborn, H.M. and McIntire, P.E., 1992. Treatment of metal‐contaminated water using bacterial sulfate reduction: Results from pilot‐scale reactors. Biotechnology and Bioengineering, 40(5), 609-616. [https://doi.org/10.1002/bit.260400508 doi 10.1002/bit.260400508]</ref><ref name= "Dreverj">Drever, J.I., The Geochemistry of Natural Waters: Surface and Groundwater Environments. Prentice-Hall, Inc., ISBN 0132727900.</ref>|| '''Fe<sup>2+</sup>''', Fe<sup>3+ </sup>|| [[File:Circle black fill.PNG|15px]]<sup>f</sup>|| [[File:Circle with diagnal line.PNG|15px]]|| [[File:Circle black fill.PNG|15px]] | + | |Iron<ref name="Dvorak1992">Dvorak, D.H., Hedin, R.S., Edenborn, H.M. and McIntire, P.E., 1992. Treatment of metal‐contaminated water using bacterial sulfate reduction: Results from pilot‐scale reactors. Biotechnology and Bioengineering, 40(5), 609-616. [https://doi.org/10.1002/bit.260400508 doi 10.1002/bit.260400508]</ref><ref name="Dreverj">Drever, J.I., The Geochemistry of Natural Waters: Surface and Groundwater Environments. Prentice-Hall, Inc., ISBN 0132727900.</ref>||'''Fe<sup>2+</sup>''', Fe<sup>3+ </sup>||[[File:Circle black fill.PNG|15px]]<sup>f</sup>||[[File:Circle with diagnal line.PNG|15px]]||[[File:Circle black fill.PNG|15px]] |
|- | |- | ||
| − | | Manganese<ref name="Dvorak1992"/><ref name= "Dreverj"/>|| '''Mn<sup>2+</sup>''', Mn<sup>4+</sup>|| [[File:Circle black fill.PNG|15px]]<sup>f</sup>|| [[File:Circle with diagnal line.PNG|15px]]|| [[File:Circle black fill.PNG|15px]] | + | |Manganese<ref name="Dvorak1992" /><ref name="Dreverj" />||'''Mn<sup>2+</sup>''', Mn<sup>4+</sup>||[[File:Circle black fill.PNG|15px]]<sup>f</sup>||[[File:Circle with diagnal line.PNG|15px]]||[[File:Circle black fill.PNG|15px]] |
|- | |- | ||
| − | | Lead, Copper, Cobalt, Nickel, Cadmium, Zinc, Mercury<ref>Blowes, D.W., Ptacek, C.J., Benner, S.G., McRae, C.W., Bennett, T.A. and Puls, R.W., 2000. Treatment of inorganic contaminants using permeable reactive barriers. Journal of Contaminant Hydrology, 45(1), 123-137.</ref><ref>Hashim, M.A., Mukhopadhyay, S., Sahu, J.N. and Sengupta, B., 2011. Remediation technologies for heavy metal contaminated groundwater. Journal of Environmental Management, 92(10), 2355-2388.</ref>|| '''Me<sup>2+</sup>'''|| NA|| NA|| [[File:Circle black fill.PNG|15px]] | + | |Lead, Copper, Cobalt, Nickel, Cadmium, Zinc, Mercury<ref>Blowes, D.W., Ptacek, C.J., Benner, S.G., McRae, C.W., Bennett, T.A. and Puls, R.W., 2000. Treatment of inorganic contaminants using permeable reactive barriers. Journal of Contaminant Hydrology, 45(1), 123-137.</ref><ref>Hashim, M.A., Mukhopadhyay, S., Sahu, J.N. and Sengupta, B., 2011. Remediation technologies for heavy metal contaminated groundwater. Journal of Environmental Management, 92(10), 2355-2388.</ref>||'''Me<sup>2+</sup>'''||NA||NA||[[File:Circle black fill.PNG|15px]] |
|- | |- | ||
| − | | colspan="12" style="color:black;text-align:left;"|Notes: [[File:Circle black fill.PNG|15px]] = Well documented immobilization mechanism; [[File:Circle with diagnal line.PNG|15px]] = Not a documented immobilization mechanism; NA = Not applicable. | + | | colspan="12" style="color:black;text-align:left;" |Notes: [[File:Circle black fill.PNG|15px]] = Well documented immobilization mechanism; [[File:Circle with diagnal line.PNG|15px]] = Not a documented immobilization mechanism; NA = Not applicable. |
|- | |- | ||
| − | | colspan="12" style="color:black;text-align:left;"|a - Found in soil and groundwater, '''bold''' indicates oxidation states of dissolved/mobile species. | + | | colspan="12" style="color:black;text-align:left;" |a - Found in soil and groundwater, '''bold''' indicates oxidation states of dissolved/mobile species. |
|- | |- | ||
| − | | colspan="12" style="color:black;text-align:left;"| b - In-situ precipitation and co-precipitation as sulfides mediated by sulfate reducing bacteria. | + | | colspan="12" style="color:black;text-align:left;" |b - In-situ precipitation and co-precipitation as sulfides mediated by sulfate reducing bacteria. |
|- | |- | ||
| − | | colspan="12" style="color:black;text-align:left;"| c - Microbial oxidation of As(III) to As(V) followed by enhanced adsorption of As(V) onto iron and manganese oxides/hydroxides. | + | | colspan="12" style="color:black;text-align:left;" |c - Microbial oxidation of As(III) to As(V) followed by enhanced adsorption of As(V) onto iron and manganese oxides/hydroxides. |
|- | |- | ||
| − | | colspan="12" style="color:black;text-align:left;"| d - Microbial reduction of Cr(VI) to less mobile Cr(III), followed by mineral precipitation and co-precipitation with Fe as oxide or oxyhydroxides. | + | | colspan="12" style="color:black;text-align:left;" |d - Microbial reduction of Cr(VI) to less mobile Cr(III), followed by mineral precipitation and co-precipitation with Fe as oxide or oxyhydroxides. |
|- | |- | ||
| − | | colspan="12" style="color:black;text-align:left;"| e - Microbial reduction, followed by precipitation and/or adsorption onto mineral phases. | + | | colspan="12" style="color:black;text-align:left;" |e - Microbial reduction, followed by precipitation and/or adsorption onto mineral phases. |
|- | |- | ||
| − | | colspan="12" style="color:black;text-align:left;"| f - Microbial oxidation to Fe<sup>3+</sup> or Mn<sup>4+</sup> followed by precipitation as oxides/hydroxides. | + | | colspan="12" style="color:black;text-align:left;" |f - Microbial oxidation to Fe<sup>3+</sup> or Mn<sup>4+</sup> followed by precipitation as oxides/hydroxides. |
|- | |- | ||
|} | |} | ||
==Technology Acceptance== | ==Technology Acceptance== | ||
| − | Bioremediation is widely applied for remediation of recalcitrant compounds present in soil and/or groundwater, and is often chosen as it can be a less expensive, adaptable to site-specific conditions, and more sustainable choice to achieve remedial goals<ref name="Stroo2010">Stroo, H.F. and Ward, C.H. eds., 2010. In situ remediation of chlorinated solvent plumes. Springer Science & Business Media. [https://doi.org/10.1007/978-1-4419-1401-9 doi 10.1007/978-1-4419-1401-9]</ref>. Multiple guidance documents are available that describe design and implementation considerations, and results from applications around the globe. This includes guidance from the United States Environmental Protection Agency (USEPA)<ref name= "USEPA2013Intro"/>, the United States Air Force, Navy and Environmental Security Technology Certification Program (ESTCP)<ref>Air Force Center for Environmental Excellence, Naval Facilities Engineering Service Center, and ESTCP, 2004. Principles and Practices of Enhanced Anaerobic Bioremediation of Chlorinated Solvents. ADA511850.</ref><ref name= "NAVFAC2015D"/>, and the Interstate Technology and Regulatory Council (ITRC)<ref name= "TRC2008">ITRC, 2008. In Situ Bioremediation of Chlorinated Ethene: DNAPL Source Zones. June, 2008.</ref>. | + | Bioremediation is widely applied for remediation of recalcitrant compounds present in soil and/or groundwater, and is often chosen as it can be a less expensive, adaptable to site-specific conditions, and more sustainable choice to achieve remedial goals<ref name="Stroo2010">Stroo, H.F. and Ward, C.H. eds., 2010. In situ remediation of chlorinated solvent plumes. Springer Science & Business Media. [https://doi.org/10.1007/978-1-4419-1401-9 doi 10.1007/978-1-4419-1401-9]</ref>. Multiple guidance documents are available that describe design and implementation considerations, and results from applications around the globe. This includes guidance from the United States Environmental Protection Agency (USEPA)<ref name="USEPA2013Intro" />, the United States Air Force, Navy and Environmental Security Technology Certification Program (ESTCP)<ref>Air Force Center for Environmental Excellence, Naval Facilities Engineering Service Center, and ESTCP, 2004. Principles and Practices of Enhanced Anaerobic Bioremediation of Chlorinated Solvents. ADA511850.</ref><ref name="NAVFAC2015D" />, and the Interstate Technology and Regulatory Council (ITRC)<ref name="TRC2008">ITRC, 2008. In Situ Bioremediation of Chlorinated Ethene: DNAPL Source Zones. June, 2008.</ref>. |
==Technology Selection and Design Considerations== | ==Technology Selection and Design Considerations== | ||
| − | The selection and [[Bioremediation - Anaerobic Design Considerations | design of an anaerobic bioremediation]] remedy should include the following factors<ref name= "USEPA2013Intro"/><ref name ="Stroo2010"/><ref name= "TRC2008"/><ref>USEPA, 2010. Green Remediation Best Management Practices: Bioremediation. EPA 542-F-10-006. [[ | + | The selection and [[Bioremediation - Anaerobic Design Considerations | design of an anaerobic bioremediation]] remedy should include the following factors<ref name="USEPA2013Intro" /><ref name="Stroo2010" /><ref name="TRC2008" /><ref>USEPA, 2010. Green Remediation Best Management Practices: Bioremediation. EPA 542-F-10-006. [[Special:FilePath/Gr factsheet biorem 32410.pdf|Report pdf]]</ref>: |
| + | |||
*'''Contaminant Treatability'''. Consider whether the target contaminants, as well as any potential co-contaminants, can be effectively treated or immobilized (in the case of metals and metalloids) by anaerobic bioremediation. | *'''Contaminant Treatability'''. Consider whether the target contaminants, as well as any potential co-contaminants, can be effectively treated or immobilized (in the case of metals and metalloids) by anaerobic bioremediation. | ||
| − | *'''Remediation of Source Zones'''. Anaerobic bioremediation has been shown a viable remedial approach for dissolved contaminant mass, and for limiting mass flux from source zones containing dense [[wikipedia: Dense non-aqueous phase liquid | non-aqueous phase liquid (DNAPL)]]. Treatment of DNAPL mass in source zones has also been demonstrated, but the remedial timeframe is typically longer than applications were aqueous phase concentrations are targeted<ref name= "TRC2008"/><ref>Cope, N. and Hughes, J.B., 2001. Biologically-enhanced removal of PCE from NAPL source zones. Environmental Science & Technology, 35(10), 2014-2021. [https://doi.org/10.1021/es0017357 doi 10.1021/es0017357]</ref><ref>Yang, Y. and McCarty, P.L., 2002. Comparison between donor substrates for biologically enhanced tetrachloroethene DNAPL dissolution. Environmental Science & Technology, 36(15), 3400-3404. [https://doi.org/10.1021/es011408e doi 10.1021/es011408e]</ref><ref>CL:AIRE, 2010. Results of Laboratory Column Studies to Determine the Potential for Bioremediation of Chlorinated Solvent DNAPL Source Areas. SABRE Bulletin 3.</ref><ref>Moretti, L., 2005. In situ bioremediation of DNAPL Source Zones. US Environmental Protection Agency, Office of Solid Waste and Emergency Response, Technology Innovation and Field Services Division.</ref>. | + | *'''Remediation of Source Zones'''. Anaerobic bioremediation has been shown a viable remedial approach for dissolved contaminant mass, and for limiting mass flux from source zones containing dense [[wikipedia: Dense non-aqueous phase liquid | non-aqueous phase liquid (DNAPL)]]. Treatment of DNAPL mass in source zones has also been demonstrated, but the remedial timeframe is typically longer than applications were aqueous phase concentrations are targeted<ref name="TRC2008" /><ref>Cope, N. and Hughes, J.B., 2001. Biologically-enhanced removal of PCE from NAPL source zones. Environmental Science & Technology, 35(10), 2014-2021. [https://doi.org/10.1021/es0017357 doi 10.1021/es0017357]</ref><ref>Yang, Y. and McCarty, P.L., 2002. Comparison between donor substrates for biologically enhanced tetrachloroethene DNAPL dissolution. Environmental Science & Technology, 36(15), 3400-3404. [https://doi.org/10.1021/es011408e doi 10.1021/es011408e]</ref><ref>CL:AIRE, 2010. Results of Laboratory Column Studies to Determine the Potential for Bioremediation of Chlorinated Solvent DNAPL Source Areas. SABRE Bulletin 3.</ref><ref>Moretti, L., 2005. In situ bioremediation of DNAPL Source Zones. US Environmental Protection Agency, Office of Solid Waste and Emergency Response, Technology Innovation and Field Services Division.</ref>. |
*'''Site Conditions'''. Low permeability and/or high heterogeneity of the targeted formation may limit amendment distribution or influence remedy design. This limitation is common to all in-situ remediation approaches and can be addressed by selection of an appropriate installation method. | *'''Site Conditions'''. Low permeability and/or high heterogeneity of the targeted formation may limit amendment distribution or influence remedy design. This limitation is common to all in-situ remediation approaches and can be addressed by selection of an appropriate installation method. | ||
*'''Incomplete biodegradation'''. Creation and/or accumulation of breakdown products (e.g., cis-1,2-dichloroethene and vinyl chloride from tetrachloroethene [PCE] and TCE) may occur. In many cases, the design of an EISB remedy can address these concerns by incorporating monitoring to confirm the effects are temporary and introducing additional amendments (pH buffers, bioaugmentation cultures) to prevent accumulation of breakdown products. | *'''Incomplete biodegradation'''. Creation and/or accumulation of breakdown products (e.g., cis-1,2-dichloroethene and vinyl chloride from tetrachloroethene [PCE] and TCE) may occur. In many cases, the design of an EISB remedy can address these concerns by incorporating monitoring to confirm the effects are temporary and introducing additional amendments (pH buffers, bioaugmentation cultures) to prevent accumulation of breakdown products. | ||
| − | *'''Potential Process Inhibition'''. Geochemical conditions (e.g., high/low pH levels) and intrinsic toxicity due to presence of elevated concentration of inhibitors such as metals, chloroform, other organic co-contaminants (e.g., 1,1,1,-trichloroethane) or sulfide should be evaluated and potential mitigation approaches considered. | + | *'''Potential Process Inhibition'''. Geochemical conditions (e.g., high/low pH levels) and intrinsic toxicity due to presence of elevated concentration of inhibitors such as metals, chloroform, other organic co-contaminants (e.g., 1,1,1,-trichloroethane) or sulfide should be evaluated and potential mitigation approaches considered. |
*[[Bioremediation - Anaerobic Secondary Water Quality Impacts |'''Secondary Effects on Water Quality''']]. Changes in pH and redox conditions in an anaerobic bioremediation zone may mobilize metals (e.g., iron, manganese, and arsenic) and form undesirable fermentation products (e.g., aldehydes and ketones). The design and monitoring program for a bioremediation remedy should account for these potential secondary effects. | *[[Bioremediation - Anaerobic Secondary Water Quality Impacts |'''Secondary Effects on Water Quality''']]. Changes in pH and redox conditions in an anaerobic bioremediation zone may mobilize metals (e.g., iron, manganese, and arsenic) and form undesirable fermentation products (e.g., aldehydes and ketones). The design and monitoring program for a bioremediation remedy should account for these potential secondary effects. | ||
*'''Volatile Byproducts'''. Stimulation of anaerobic biodegradation may enhance generation of gases (e.g., vinyl chloride, methane, or hydrogen sulfide) that may degrade groundwater quality and/or accumulate in the vadose zone. Optimization of the EISB remedy can mitigate and monitor these effects (e.g., use of lower electron donor rates to limit methanogenesis, use of bioaugmentation to prevent vinyl chloride formation, and use of soil gas monitoring). | *'''Volatile Byproducts'''. Stimulation of anaerobic biodegradation may enhance generation of gases (e.g., vinyl chloride, methane, or hydrogen sulfide) that may degrade groundwater quality and/or accumulate in the vadose zone. Optimization of the EISB remedy can mitigate and monitor these effects (e.g., use of lower electron donor rates to limit methanogenesis, use of bioaugmentation to prevent vinyl chloride formation, and use of soil gas monitoring). | ||
| Line 121: | Line 122: | ||
==Biostimulation== | ==Biostimulation== | ||
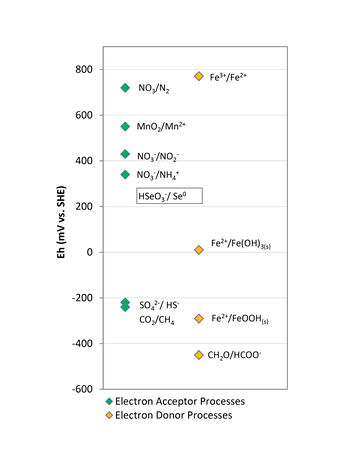
[[File:MacKinnon AnBiorem Fig1.jpg|thumbnail|350px|left|Figure 1. Redox ladder for common electron donors and electron acceptors.]] | [[File:MacKinnon AnBiorem Fig1.jpg|thumbnail|350px|left|Figure 1. Redox ladder for common electron donors and electron acceptors.]] | ||
| − | Biostimulation means adding compounds to the subsurface to encourage indigenous microorganisms to metabolize target contaminants<ref name= "USEPA2013Intro"/>. In anaerobic reductive processes, simple organic carbon compounds (e.g., sugars, alcohols, aliphatic hydrocarbons, and volatile fatty acids) serve as electron donors to stimulate anaerobic bacterial growth, and thus enhance the rate and extent of biodegradation of the target contaminants. For anaerobic oxidative processes, it may be necessary to add electron acceptors, such as nitrate or sulfate to enhance biodegradation. A crucial component of the [[Bioremediation - Anaerobic Design Considerations | design]] of anaerobic bioremediation systems is selection of appropriate amendments, and their application dosages. | + | Biostimulation means adding compounds to the subsurface to encourage indigenous microorganisms to metabolize target contaminants<ref name="USEPA2013Intro" />. In anaerobic reductive processes, simple organic carbon compounds (e.g., sugars, alcohols, aliphatic hydrocarbons, and volatile fatty acids) serve as electron donors to stimulate anaerobic bacterial growth, and thus enhance the rate and extent of biodegradation of the target contaminants. For anaerobic oxidative processes, it may be necessary to add electron acceptors, such as nitrate or sulfate to enhance biodegradation. A crucial component of the [[Bioremediation - Anaerobic Design Considerations | design]] of anaerobic bioremediation systems is selection of appropriate amendments, and their application dosages. |
In the case of electron donors, the amendments used in bioremediation applications are typically classified as quick release compounds (lactate, sodium benzoate, molasses, whey) or slow release compounds (emulsified vegetable oils, HRC<sup>®</sup>, EHC<sup>®</sup>, ABC<sup>®</sup>+, mulch, compost). These electron donors are added in anaerobic bioremediation to stimulate conditions conducive to the degradation processes by depleting the dissolved oxygen (DO), and other terminal electron acceptors, and lowering the oxidation-reduction potential of groundwater. In addition, products of electron donor fermentation (i.e., simple organic acids, hydrogen) are required as an energy source for metabolism of decontaminating microbes. The amount of energy released during electron transfer from the donor is controlled by the redox potential (Eh) of the terminal electron acceptor. Therefore, anaerobic microorganisms typically use available native electron acceptors in the following order of preference: nitrate, manganese and ferric iron oxyhydroxides, sulfate, and finally carbon dioxide (Fig. 1). | In the case of electron donors, the amendments used in bioremediation applications are typically classified as quick release compounds (lactate, sodium benzoate, molasses, whey) or slow release compounds (emulsified vegetable oils, HRC<sup>®</sup>, EHC<sup>®</sup>, ABC<sup>®</sup>+, mulch, compost). These electron donors are added in anaerobic bioremediation to stimulate conditions conducive to the degradation processes by depleting the dissolved oxygen (DO), and other terminal electron acceptors, and lowering the oxidation-reduction potential of groundwater. In addition, products of electron donor fermentation (i.e., simple organic acids, hydrogen) are required as an energy source for metabolism of decontaminating microbes. The amount of energy released during electron transfer from the donor is controlled by the redox potential (Eh) of the terminal electron acceptor. Therefore, anaerobic microorganisms typically use available native electron acceptors in the following order of preference: nitrate, manganese and ferric iron oxyhydroxides, sulfate, and finally carbon dioxide (Fig. 1). | ||
| Line 129: | Line 130: | ||
==Bioaugmentation== | ==Bioaugmentation== | ||
[[File:MacKinnon AnBiorem Fig2.jpg|thumbnail|right|Figure 2. Example of bioaugmentation at a field site.]] | [[File:MacKinnon AnBiorem Fig2.jpg|thumbnail|right|Figure 2. Example of bioaugmentation at a field site.]] | ||
| − | Bioaugmentation may be considered at a site when an appropriate population of anaerobic microorganisms is not present or sufficiently active to stimulate complete anaerobic degradation of the existing contaminants. While microorganisms necessary for complete biodegradation of some contaminants (i.e., perchlorate) can be fairly widespread, this is not always the case. In these cases, bioaugmentation is used to enhance bioremediation. Bioaugmentation involves the injection of microbial cultures comprised of non-native organisms known to degrade the targeted contaminants to completion (Fig. 2). For example, the presence of Dehalococcoides-related microorganisms has been linked to complete dechlorination of PCE and TCE to ethene in the field<ref>Parsons, 2004. Principles and Practices of Enhanced Anaerobic Bioremediation of Chlorinated Solvents. AFCEE, NFEC, ESTCP. [ | + | Bioaugmentation may be considered at a site when an appropriate population of anaerobic microorganisms is not present or sufficiently active to stimulate complete anaerobic degradation of the existing contaminants. While microorganisms necessary for complete biodegradation of some contaminants (i.e., perchlorate) can be fairly widespread, this is not always the case. In these cases, bioaugmentation is used to enhance bioremediation. Bioaugmentation involves the injection of microbial cultures comprised of non-native organisms known to degrade the targeted contaminants to completion (Fig. 2). For example, the presence of Dehalococcoides-related microorganisms has been linked to complete dechlorination of PCE and TCE to ethene in the field<ref>Parsons, 2004. Principles and Practices of Enhanced Anaerobic Bioremediation of Chlorinated Solvents. AFCEE, NFEC, ESTCP. [//www.enviro.wiki/images/d/d5/AFCEE_Principles_and_Practices.pdf Report pdf]</ref><ref>Major, D.W., McMaster, M.L., Cox, E.E., Edwards, E.A., Dworatzek, S.M., Hendrickson, E.R., Starr, M.G., Payne, J.A. and Buonamici, L.W., 2002. Field demonstration of successful bioaugmentation to achieve dechlorination of tetrachloroethene to ethene. Environmental Science & Technology, 36(23), 5106-5116. [https://doi.org/10.1021/es0255711 doi 10.1021/es0255711]</ref><ref>Hendrickson, E.R., Payne, J.A., Young, R.M., Starr, M.G., Perry, M.P., Fahnestock, S., Ellis, D.E. and Ebersole, R.C., 2002. Molecular analysis of Dehalococcoides 16S ribosomal DNA from chloroethene-contaminated sites throughout North America and Europe. Applied and Environmental Microbiology, 68(2), 485-495. [https://doi.org/10.1128/aem.68.2.485-495.2002 doi 10.1128/aem.68.2.485-495.2002]</ref><ref>Lendvay, J.M., Löffler, F.E., Dollhopf, M., Aiello, M.R., Daniels, G., Fathepure, B.Z., Gebhard, M., Heine, R., Helton, R., Shi, J., Krajmalnik-Brown, R., Major Jr., C.L., Barcelona, M.J., Petrovskis, E., Hickey, R., Tiedje, J.M., Adriaens, P., 2003. Bioreactive barriers: a comparison of bioaugmentation and biostimulation for chlorinated solvent remediation. Environmental Science & Technology, 37(7), 1422-1431. [https://doi.org/10.1021/es025985u doi 10.1021/es025985u]</ref>. Commercially available bioaugmentation products that contain these microorganisms include KB-1<sup>®</sup>, SDC-9™, and Bio-Dechlor Inoculum<sup>®</sup> Plus. |
==Summary== | ==Summary== | ||
| Line 135: | Line 136: | ||
There have been numerous field demonstrations of anaerobic bioremediation documented in publicly available literature and reports, including: | There have been numerous field demonstrations of anaerobic bioremediation documented in publicly available literature and reports, including: | ||
| + | |||
*U.S. EPA Contaminated Site Clean-Up Information (CLU-IN)<ref>USEPA 2016. Anaerobic Bioremediation (Direct) Application.</ref> | *U.S. EPA Contaminated Site Clean-Up Information (CLU-IN)<ref>USEPA 2016. Anaerobic Bioremediation (Direct) Application.</ref> | ||
| − | *In Situ Bioremediation of Chlorinated Ethene DNAPL Source Zones: Case Studies<ref name= "ITRC2007">ITRC, 2007. In Situ Bioremediation of Chlorinated Ethene DNAPL Source Zones: Case Studies, BioDNAPL-2, 173 pp, 2007.[ | + | *In Situ Bioremediation of Chlorinated Ethene DNAPL Source Zones: Case Studies<ref name="ITRC2007">ITRC, 2007. In Situ Bioremediation of Chlorinated Ethene DNAPL Source Zones: Case Studies, BioDNAPL-2, 173 pp, 2007.[//www.enviro.wiki/images/5/5a/ITRC-2007-Bioremed_of_Chlorinated_Ethene.pdf Report pdf]</ref> |
*ESTCP Demonstrations: Cost and Performance Reports | *ESTCP Demonstrations: Cost and Performance Reports | ||
**ER-0008 - Biodegradation of Dense Non-Aqueous Phase Liquids (DNAPLs) through Bioaugmentation of Source Areas - Dover National Test Site<ref>ESTCP, 2008. Biodegradation of Dense Non-Aqueous Phase Liquids (DNAPLs) through Bioaugmentation of Source Areas - Dover National Test Site, Dover, Delaware: ESTCP Cost and Performance Report. [https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Persistent-Contamination/ER-200008/ER-200008 ESTCP Project ER-0008]</ref> | **ER-0008 - Biodegradation of Dense Non-Aqueous Phase Liquids (DNAPLs) through Bioaugmentation of Source Areas - Dover National Test Site<ref>ESTCP, 2008. Biodegradation of Dense Non-Aqueous Phase Liquids (DNAPLs) through Bioaugmentation of Source Areas - Dover National Test Site, Dover, Delaware: ESTCP Cost and Performance Report. [https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Persistent-Contamination/ER-200008/ER-200008 ESTCP Project ER-0008]</ref> | ||
| − | **ER-0221 - Edible Oil Barriers for Treatment of Chlorinated Solvent Groundwater<ref>Lieberman, M.T. and Borden, R.C., 2009. Edible Oil Barriers for Treatment of Chlorinated Solvent Contaminated Groundwater. Solutions Industrial and Environmental Services Raleigh, NC. [ | + | **ER-0221 - Edible Oil Barriers for Treatment of Chlorinated Solvent Groundwater<ref>Lieberman, M.T. and Borden, R.C., 2009. Edible Oil Barriers for Treatment of Chlorinated Solvent Contaminated Groundwater. Solutions Industrial and Environmental Services Raleigh, NC. [//www.enviro.wiki/images/4/45/PRB-ER-0221-FR.pdf Report pdf]</ref> |
| − | **ER-200219 - Comparative Demonstration of Active and Semi-Passive In Situ Bioremediation Approaches for Perchlorate Impacted Groundwater: Active In Situ Bioremediation Demonstration (Aerojet Facility)<ref name= "Cox2012">Cox, E. and Krug, T., 2012. Comparative Demonstration of Active and Semi-Passive In Situ Bioremediation Approaches for Perchlorate Impacted Groundwater: Active In Situ Bioremediation Demonstration (Aerojet Facility) ESTCP Project ER-200219. [https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Emerging-Issues/ER-200219 ER-200219]</ref> | + | **ER-200219 - Comparative Demonstration of Active and Semi-Passive In Situ Bioremediation Approaches for Perchlorate Impacted Groundwater: Active In Situ Bioremediation Demonstration (Aerojet Facility)<ref name="Cox2012">Cox, E. and Krug, T., 2012. Comparative Demonstration of Active and Semi-Passive In Situ Bioremediation Approaches for Perchlorate Impacted Groundwater: Active In Situ Bioremediation Demonstration (Aerojet Facility) ESTCP Project ER-200219. [https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Emerging-Issues/ER-200219 ER-200219]</ref> |
| − | **ER-200627 - Loading Rate and Impacts of Substrate Delivery for Enhanced Anaerobic Bioremediation<ref name= "ESTCP2010LR">Henry, B., 2010. Loading Rate and Impacts of Substrate Delivery for Enhanced Anaerobic Bioremediation. ESTCP Project ER-200627, 90 pgs. [https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200627 ER-200627]</ref> | + | **ER-200627 - Loading Rate and Impacts of Substrate Delivery for Enhanced Anaerobic Bioremediation<ref name="ESTCP2010LR">Henry, B., 2010. Loading Rate and Impacts of Substrate Delivery for Enhanced Anaerobic Bioremediation. ESTCP Project ER-200627, 90 pgs. [https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200627 ER-200627]</ref> |
| − | *Regulatory / Guidance Documents<ref name= "ITRC2007"/><ref name= "Cox2012"/><ref name= "ESTCP2010LR"/><ref>Robinson, C., Barry, D.A., McCarty, P.L., Gerhard, J.I. and Kouznetsova, I., 2009. pH control for enhanced reductive bioremediation of chlorinated solvent source zones. Science of the Total Environment, 407(16), 4560-4573. [https://doi.org/10.1016/j.scitotenv.2009.03.029 doi 10.1016/j.scitotenv.2009.03.029]</ref><ref>Borden, R.C., Cha, K.Y., Simpkin, T. and Lieberman, M.T., 2012. Development of a Design Tool for Planning Aqueous Amendment Injection Systems Soluble Substrate Design Tool (No. ER-200626). North Carolina State Univ. at Raleigh. [https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200626/ER-200626 Report pdf]</ref> | + | *Regulatory / Guidance Documents<ref name="ITRC2007" /><ref name="Cox2012" /><ref name="ESTCP2010LR" /><ref>Robinson, C., Barry, D.A., McCarty, P.L., Gerhard, J.I. and Kouznetsova, I., 2009. pH control for enhanced reductive bioremediation of chlorinated solvent source zones. Science of the Total Environment, 407(16), 4560-4573. [https://doi.org/10.1016/j.scitotenv.2009.03.029 doi 10.1016/j.scitotenv.2009.03.029]</ref><ref>Borden, R.C., Cha, K.Y., Simpkin, T. and Lieberman, M.T., 2012. Development of a Design Tool for Planning Aqueous Amendment Injection Systems Soluble Substrate Design Tool (No. ER-200626). North Carolina State Univ. at Raleigh. [https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200626/ER-200626 Report pdf]</ref> |
On-going research and development for bioremediation includes the following SERDP / ESTCP projects: | On-going research and development for bioremediation includes the following SERDP / ESTCP projects: | ||
| + | |||
*ER-201428 - Long-Term Performance Assessment at a Highly Characterized and Instrumented DNAPL Source Area following Bioaugmentation | *ER-201428 - Long-Term Performance Assessment at a Highly Characterized and Instrumented DNAPL Source Area following Bioaugmentation | ||
*ER-2311 - Development of an Integrated Field Test/Modeling Protocol for Efficient In Situ Bioremediation Design and Performance Uncertainty Assessment | *ER-2311 - Development of an Integrated Field Test/Modeling Protocol for Efficient In Situ Bioremediation Design and Performance Uncertainty Assessment | ||
| Line 156: | Line 159: | ||
==References== | ==References== | ||
| − | <references/> | + | <references /> |
==See Also== | ==See Also== | ||
| + | |||
*[https://clu-in.org/techfocus/default.focus/sec/Bioremediation/cat/Anaerobic_Bioremediation_(Direct)/p/2 Bioremediation] | *[https://clu-in.org/techfocus/default.focus/sec/Bioremediation/cat/Anaerobic_Bioremediation_(Direct)/p/2 Bioremediation] | ||
*[https://clu-in.org/download/contaminantfocus/dnapl/Treatment_Technologies/epa_2006_engin_issue_bio.pdf In Situ and Ex Situ Biodegradation Technologies for Remediation of Contaminated Sites] | *[https://clu-in.org/download/contaminantfocus/dnapl/Treatment_Technologies/epa_2006_engin_issue_bio.pdf In Situ and Ex Situ Biodegradation Technologies for Remediation of Contaminated Sites] | ||
*[https://clu-in.org/download/remed/Bioaug2005.pdf Bioaugmentation For Remediation of Chlorinated Solvents] | *[https://clu-in.org/download/remed/Bioaug2005.pdf Bioaugmentation For Remediation of Chlorinated Solvents] | ||
*[https://frtr.gov/costperformance/pdf/remediation/principles_and_practices_bioremediation.pdf Principles and Practices of Enhanced Anaerobic Bioremediation of Chlorinated Solvents] | *[https://frtr.gov/costperformance/pdf/remediation/principles_and_practices_bioremediation.pdf Principles and Practices of Enhanced Anaerobic Bioremediation of Chlorinated Solvents] | ||
| − | *[http://www.itrcweb.org/Team/Public?teamID=23 Bioremediation of DNAPLs] | + | *[http://www.itrcweb.org/Team/Public?teamID=23 Bioremediation of DNAPLs] |
| − | *[http://www.itrcweb.org/Team/Public?teamID=33 In Situ Bioremediation] | + | *[http://www.itrcweb.org/Team/Public?teamID=33 In Situ Bioremediation] |
*[http://navfac.navy.mil/content/dam/navfac/Specialty%20Centers/Engineering%20and%20Expeditionary%20Warfare%20Center/Environmental/Restoration/er_pdfs/b/navfac-ev-fs-biorem-dnapl-20120412.pdf Using Bioremediation in Dense Non-Aqueous Phase Liquid Source Zones] | *[http://navfac.navy.mil/content/dam/navfac/Specialty%20Centers/Engineering%20and%20Expeditionary%20Warfare%20Center/Environmental/Restoration/er_pdfs/b/navfac-ev-fs-biorem-dnapl-20120412.pdf Using Bioremediation in Dense Non-Aqueous Phase Liquid Source Zones] | ||
*[http://navfac.navy.mil/content/dam/navfac/Specialty%20Centers/Engineering%20and%20Expeditionary%20Warfare%20Center/Environmental/Restoration/er_pdfs/d/navfacexwc-ev-tm-1501-erd-design-201503f.pdf Design Considerations for Enhanced Reductive Dechlorination] | *[http://navfac.navy.mil/content/dam/navfac/Specialty%20Centers/Engineering%20and%20Expeditionary%20Warfare%20Center/Environmental/Restoration/er_pdfs/d/navfacexwc-ev-tm-1501-erd-design-201503f.pdf Design Considerations for Enhanced Reductive Dechlorination] | ||
*[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-1177 Novel Approach for Stimulating Reductive Dechlorination of Solvents] | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-1177 Novel Approach for Stimulating Reductive Dechlorination of Solvents] | ||
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Persistent-Contamination/ER-1203 | + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Persistent-Contamination/ER-1203 Foam Delivery of Hydrogen for Enhanced Aquifer Contacting and Anaerobic Bioremediation of Chlorinated Solvents] |
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-1205 | + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-1205 Development of Permeable Reactive Barriers Using Edible Oils] |
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-1206 | + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-1206 Low-Volume Pulsed Biosparging of Hydrogen for Bioremediation of Chlorinated Solvent Plumes] |
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-199914 | + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-199914 Demonstration of Bioaugmentation at Kelly Air Force Base, Texas] |
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-199719 | + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-199719 Reductive Anaerobic Biological In Situ Treatment Technology (RABITT) Treatability Testing] |
*[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-199920 Molasses-Induced Reactive Zones to Treat Chlorinated Hydrocarbons] | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-199920 Molasses-Induced Reactive Zones to Treat Chlorinated Hydrocarbons] | ||
*[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200125 Evaluation of Performance and Costs Associated with Anaerobic Dechlorination] | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200125 Evaluation of Performance and Costs Associated with Anaerobic Dechlorination] | ||
*[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200221 Edible Oil Barriers for Treatment of Chlorinated Solvent- and Perchlorate-Contaminated Groundwater] | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200221 Edible Oil Barriers for Treatment of Chlorinated Solvent- and Perchlorate-Contaminated Groundwater] | ||
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200429 | + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200429 Field Comparison of Biofouling Control Measures for In Situ Bioremediation of Groundwater] |
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Persistent-Contamination/ER-200438 | + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Persistent-Contamination/ER-200438 Reductions in DNAPL Longevity Through Biological Flux Enhancement] |
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200513 | + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200513 A Low-Cost, Passive Approach for Bacterial Growth and Distribution for Large-Scale Implementation of Bioaugmentation] |
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200515 | + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200515 Bioaugmentation for Groundwater Remediation] |
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200626 | + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200626 Development of Design Tools for Planning Aqueous Amendment Injection Systems] |
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200627 | + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200627 Loading Rates and Impacts of Substrate Delivery for Enhanced Anaerobic Bioremediation] |
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Persistent-Contamination/ER-200716 | + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Persistent-Contamination/ER-200716 Improving Effectiveness of Bioremediation at DNAPL Source Zone Sites Applying Partitioning Electron Donors] |
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Persistent-Contamination/ER-200218 | + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Persistent-Contamination/ER-200218 In Situ Bioremediation of Chlorinated Solvents Source Areas with Enhanced Mass Transfer] |
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-201027 | + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-201027 Enhanced Attenuation of Unsaturated Chlorinated Solvent Source Zones Using Direct Hydrogen Delivery] |
Latest revision as of 02:47, 28 April 2022
Bioremediation is the process by which contaminants in soil and/or groundwater are treated biologically, primarily by microorganisms or biomolecules generated by the cells. Bioremediation processes can take place under oxic (with oxygen) or anoxic (without oxygen) conditions. This article focuses on enhanced in situ bioremediation (EISB) for the anaerobic biodegradation of organic contaminants, particularly chlorinated solvents, in soil and groundwater. However, much of the information provided is applicable to other contaminant types. EISB is frequently selected as a remedial technology as it can provide complete degradation of contaminants utilizing natural microbial processes, is able to be implemented in a variety of site conditions, and is relatively low cost compared to more active engineered remedial systems.
Related Article(s):
- Bioremediation - Anaerobic Design Considerations
- Bioremediation - Anaerobic Secondary Water Quality Impacts
- Design Tool - Base Addition for ERD
- Low pH Inhibition of Reductive Dechlorination
Contributor(s): Michaye McMaster and Leah MacKinnon, M.A.Sc., P.E.
Key Resource(s):
- Introduction to In Situ Bioremediation of Groundwater. EPA 542-R-13-018[1]
- Design Considerations for Enhanced Reductive Dechlorination. TM-NAVFAC-EXWC-EV-1501[2]
Introduction
Laboratory and field applications over the past two decades have shown that microorganisms in subsurface environments can degrade a wide variety of chemicals to environmentally acceptable end products. Anaerobic bioremediation of contaminated groundwater and geologic materials typically involves in-situ treatment via biostimulation using various carbon-based amendments, because most sites lack sufficient organic carbon to promote anaerobic microbial respiration. In some cases, bioaugmentation, the injection of a microbial culture, may be required to provide the appropriate microbial community to promote complete degradation of the target contaminants. Anaerobic bioremediation remedies typically involve an initial amendment application, followed by a period of monitoring to demonstrate the remedial goals have been achieved and to evaluate the need for additional amendment applications.
Degradation Processes
Under anaerobic conditions, organic contaminants can serve as the electron acceptors or electron donors during biodegradation processes[1]; we refer to the former as “anaerobic reductive bioremediation” and the latter as “anaerobic oxidative bioremediation”.
- Anaerobic reductive bioremediation relies on the presence of biologically available organic carbon, which may be naturally present or added to stimulate biological activity. Organic bioremediation amendments, referred to as organic substrates or electron donors, generate and sustain anoxic conditions by consuming oxygen via aerobic respiration, as well as other electron acceptors, during its biodegradation. For example, chlorinated solvents such as trichloroethene (TCE) serve as electron acceptors and undergo reductive dechlorination under anaerobic conditions in the presence of an electron donor. This microbial-mediated process can result in the complete degradation of many specific chlorinated solvents to innocuous end products.
- Anaerobic oxidative bioremediation relies on other electron acceptors such as nitrate or sulfate for direct microbial metabolic oxidation of a contaminant serving as the electron donor. This approach may be applied for the treatment of non-chlorinated hydrocarbon compounds where oxygen has already been depleted.
In contrast, cometabolism occurs when microorganisms do not utilize the organic contaminant as an energy source, but the contaminant is fortuitously degraded by enzymes or co-factors produced during the metabolism of another compound.
Contaminant Treatability
Many contaminants can be degraded by bioremediation in both laboratory and field settings including the use of biological treatment processes for common organic contaminants as well as metals, metalloids, and perchlorate (Tables 1, 2).
| Contaminant | Aerobic Oxidation | Aerobic Cometabolism | Anaerobic Oxidation | Anaerobic Reduction | Cometabolic Anaerobic Reduction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Perchlorate[1][3] | |||||||||||
| TNT/RDX/HMX[4][5][6] | |||||||||||
| 1,4-dioxane[7][8][9] | |||||||||||
| Chloroethenes[10][11] | |||||||||||
| Chloroethanes[10][11] | |||||||||||
| Chloromethanes[10][11][12] | |||||||||||
| Chlorobenzenes[13][14][15] | |||||||||||
| Nitrobenzenes[5][13] | |||||||||||
| BTEX[10][11][16] | |||||||||||
| MTBE and TBA[17][18] | |||||||||||
| Chloropropanes[12][19] | |||||||||||
| Notes: | |||||||||||
| Metal/Metalloid | Oxidation Statesa | Aerobic oxidation | Anaerobic reduction | ISP via SRBb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxyanions | |||||||||||
| Arsenic[20][21][22] | As (III, V) | ||||||||||
| Chromium[23][24] | Cr (III, VI) | ||||||||||
| Selenium[25] | Se (-II, 0, IV, VI) | ||||||||||
| Uranium[26][27] | U (IV, VI) | ||||||||||
| Metal Cations Notes: | |||||||||||
| Iron[28][29] | Fe2+, Fe3+ | ||||||||||
| Manganese[28][29] | Mn2+, Mn4+ | ||||||||||
| Lead, Copper, Cobalt, Nickel, Cadmium, Zinc, Mercury[30][31] | Me2+ | NA | NA | ||||||||
| Notes: | |||||||||||
| a - Found in soil and groundwater, bold indicates oxidation states of dissolved/mobile species. | |||||||||||
| b - In-situ precipitation and co-precipitation as sulfides mediated by sulfate reducing bacteria. | |||||||||||
| c - Microbial oxidation of As(III) to As(V) followed by enhanced adsorption of As(V) onto iron and manganese oxides/hydroxides. | |||||||||||
| d - Microbial reduction of Cr(VI) to less mobile Cr(III), followed by mineral precipitation and co-precipitation with Fe as oxide or oxyhydroxides. | |||||||||||
| e - Microbial reduction, followed by precipitation and/or adsorption onto mineral phases. | |||||||||||
| f - Microbial oxidation to Fe3+ or Mn4+ followed by precipitation as oxides/hydroxides. | |||||||||||
Technology Acceptance
Bioremediation is widely applied for remediation of recalcitrant compounds present in soil and/or groundwater, and is often chosen as it can be a less expensive, adaptable to site-specific conditions, and more sustainable choice to achieve remedial goals[32]. Multiple guidance documents are available that describe design and implementation considerations, and results from applications around the globe. This includes guidance from the United States Environmental Protection Agency (USEPA)[1], the United States Air Force, Navy and Environmental Security Technology Certification Program (ESTCP)[33][2], and the Interstate Technology and Regulatory Council (ITRC)[34].
Technology Selection and Design Considerations
The selection and design of an anaerobic bioremediation remedy should include the following factors[1][32][34][35]:
- Contaminant Treatability. Consider whether the target contaminants, as well as any potential co-contaminants, can be effectively treated or immobilized (in the case of metals and metalloids) by anaerobic bioremediation.
- Remediation of Source Zones. Anaerobic bioremediation has been shown a viable remedial approach for dissolved contaminant mass, and for limiting mass flux from source zones containing dense non-aqueous phase liquid (DNAPL). Treatment of DNAPL mass in source zones has also been demonstrated, but the remedial timeframe is typically longer than applications were aqueous phase concentrations are targeted[34][36][37][38][39].
- Site Conditions. Low permeability and/or high heterogeneity of the targeted formation may limit amendment distribution or influence remedy design. This limitation is common to all in-situ remediation approaches and can be addressed by selection of an appropriate installation method.
- Incomplete biodegradation. Creation and/or accumulation of breakdown products (e.g., cis-1,2-dichloroethene and vinyl chloride from tetrachloroethene [PCE] and TCE) may occur. In many cases, the design of an EISB remedy can address these concerns by incorporating monitoring to confirm the effects are temporary and introducing additional amendments (pH buffers, bioaugmentation cultures) to prevent accumulation of breakdown products.
- Potential Process Inhibition. Geochemical conditions (e.g., high/low pH levels) and intrinsic toxicity due to presence of elevated concentration of inhibitors such as metals, chloroform, other organic co-contaminants (e.g., 1,1,1,-trichloroethane) or sulfide should be evaluated and potential mitigation approaches considered.
- Secondary Effects on Water Quality. Changes in pH and redox conditions in an anaerobic bioremediation zone may mobilize metals (e.g., iron, manganese, and arsenic) and form undesirable fermentation products (e.g., aldehydes and ketones). The design and monitoring program for a bioremediation remedy should account for these potential secondary effects.
- Volatile Byproducts. Stimulation of anaerobic biodegradation may enhance generation of gases (e.g., vinyl chloride, methane, or hydrogen sulfide) that may degrade groundwater quality and/or accumulate in the vadose zone. Optimization of the EISB remedy can mitigate and monitor these effects (e.g., use of lower electron donor rates to limit methanogenesis, use of bioaugmentation to prevent vinyl chloride formation, and use of soil gas monitoring).
- Cost. Amendment material and implementation cost as compared to other viable remediation approaches (e.g., in situ chemical reduction (ISCR), in situ chemical oxidation (ISCO)).
- Timeframe for Remediation. Development of anaerobic conditions, and the microbial populations capable of complete treatment of target contaminants, may require several months to years. While bioremediation may take longer than other remedial approaches (e.g., ISCO, in situ thermal remediation), this is frequently balanced by lower cost, higher sustainability and reduced likelihood of rebound following remediation.
- Sustainability. Anaerobic bioremediation frequently uses less electricity and water than many other remedial alternatives, and uses non-toxic amendments, which make it desirable as a sustainable remedial tool. In situ applications avoid transport of materials off-site to disposal facilities.
Biostimulation
Biostimulation means adding compounds to the subsurface to encourage indigenous microorganisms to metabolize target contaminants[1]. In anaerobic reductive processes, simple organic carbon compounds (e.g., sugars, alcohols, aliphatic hydrocarbons, and volatile fatty acids) serve as electron donors to stimulate anaerobic bacterial growth, and thus enhance the rate and extent of biodegradation of the target contaminants. For anaerobic oxidative processes, it may be necessary to add electron acceptors, such as nitrate or sulfate to enhance biodegradation. A crucial component of the design of anaerobic bioremediation systems is selection of appropriate amendments, and their application dosages.
In the case of electron donors, the amendments used in bioremediation applications are typically classified as quick release compounds (lactate, sodium benzoate, molasses, whey) or slow release compounds (emulsified vegetable oils, HRC®, EHC®, ABC®+, mulch, compost). These electron donors are added in anaerobic bioremediation to stimulate conditions conducive to the degradation processes by depleting the dissolved oxygen (DO), and other terminal electron acceptors, and lowering the oxidation-reduction potential of groundwater. In addition, products of electron donor fermentation (i.e., simple organic acids, hydrogen) are required as an energy source for metabolism of decontaminating microbes. The amount of energy released during electron transfer from the donor is controlled by the redox potential (Eh) of the terminal electron acceptor. Therefore, anaerobic microorganisms typically use available native electron acceptors in the following order of preference: nitrate, manganese and ferric iron oxyhydroxides, sulfate, and finally carbon dioxide (Fig. 1).
Reductive dechlorination of more highly halogenated organics such as PCE and TCE to dichloroethene (DCE) can occur under mildly reducing nitrate or iron reducing conditions[40]; however, the complete reductive dechlorination of the widest range of the targeted compounds (including degradation of DCE to ethene) often occurs under more strongly reducing conditions of sulfate reduction or methanogenesis[41]. Lightly halogenated organics such as vinyl chloride have also been demonstrated to undergo anaerobic oxidation under iron reducing conditions[42]. Therefore, the optimal anaerobic conditions for complete dechlorination occurs after the competing electron acceptors such as DO, nitrate, and manganese are consumed.
Bioaugmentation
Bioaugmentation may be considered at a site when an appropriate population of anaerobic microorganisms is not present or sufficiently active to stimulate complete anaerobic degradation of the existing contaminants. While microorganisms necessary for complete biodegradation of some contaminants (i.e., perchlorate) can be fairly widespread, this is not always the case. In these cases, bioaugmentation is used to enhance bioremediation. Bioaugmentation involves the injection of microbial cultures comprised of non-native organisms known to degrade the targeted contaminants to completion (Fig. 2). For example, the presence of Dehalococcoides-related microorganisms has been linked to complete dechlorination of PCE and TCE to ethene in the field[43][44][45][46]. Commercially available bioaugmentation products that contain these microorganisms include KB-1®, SDC-9™, and Bio-Dechlor Inoculum® Plus.
Summary
Anaerobic bioremediation is a well-demonstrated remediation strategy for the treatment of a wide range of organic contaminants, most notably chlorinated solvents. The injection of carbon-based electron donors for biostimulation and microbial cultures for bioaugmentation can promote the complete anaerobic biodegradation of contaminants in soil and groundwater. Anaerobic bioremediation has also been used to treat metals and metalloids via immobilization processes.
There have been numerous field demonstrations of anaerobic bioremediation documented in publicly available literature and reports, including:
- U.S. EPA Contaminated Site Clean-Up Information (CLU-IN)[47]
- In Situ Bioremediation of Chlorinated Ethene DNAPL Source Zones: Case Studies[48]
- ESTCP Demonstrations: Cost and Performance Reports
- ER-0008 - Biodegradation of Dense Non-Aqueous Phase Liquids (DNAPLs) through Bioaugmentation of Source Areas - Dover National Test Site[49]
- ER-0221 - Edible Oil Barriers for Treatment of Chlorinated Solvent Groundwater[50]
- ER-200219 - Comparative Demonstration of Active and Semi-Passive In Situ Bioremediation Approaches for Perchlorate Impacted Groundwater: Active In Situ Bioremediation Demonstration (Aerojet Facility)[51]
- ER-200627 - Loading Rate and Impacts of Substrate Delivery for Enhanced Anaerobic Bioremediation[52]
- Regulatory / Guidance Documents[48][51][52][53][54]
On-going research and development for bioremediation includes the following SERDP / ESTCP projects:
- ER-201428 - Long-Term Performance Assessment at a Highly Characterized and Instrumented DNAPL Source Area following Bioaugmentation
- ER-2311 - Development of an Integrated Field Test/Modeling Protocol for Efficient In Situ Bioremediation Design and Performance Uncertainty Assessment
- ER-2312 - Advanced Environmental Molecular Diagnostics to Assess, Monitor, and Predict Microbial Activities at Complicated Chlorinated Solvent Sites
- ER-201325 - Electrokinetic-Enhanced (EK-Enhanced) Amendment Delivery for Remediation of Low Permeability and Heterogeneous Materials
- ER-2530- Biogeochemical Processes that Control Natural Attenuation of Trichloroethylene in Low Permeability Zones
- ER-2532 - Biologically Mediated Abiotic Degradation of Chlorinated Ethenes: A New Conceptual Framework
- ER-201581 - Post-Remediation Evaluation of EVO Treatment - How Can We Improve Performance?
- ER-201629 - Evaluation of a Sustainable and Passive Approach to Treat Large, Dilute Chlorinated VOC Groundwater Plumes
References
- ^ 1.0 1.1 1.2 1.3 1.4 1.5 USEPA, 2013. Introduction to In Situ Bioremediation of Groundwater. EPA 542-R-13-018. Report pdf
- ^ 2.0 2.1 NAVFAC, 2015. Design considerations for Enhanced Reductive Dechlorination. TM-NAVFAC-EXWC-EV-1501. Report pdf
- ^ Stroo, H.F., Loehr, R.C., Ward, C.H. eds., 2008. In situ bioremediation of perchlorate in groundwater. Springer Science & Business Media. doi: 10.1007/978-0-387-84921-8_10
- ^ Spain, J.C., Hughes, J.B. and Knackmuss, H.J. eds., 2000. Biodegradation of nitroaromatic compounds and explosives. CRC Press
- ^ 5.0 5.1 Kalderis, D., Juhasz, A.L., Boopathy, R. and Comfort, S., 2011. Soils contaminated with explosives: Environmental fate and evaluation of state-of-the-art remediation processes (IUPAC Technical Report). Pure and Applied Chemistry, 83(7), 1407-1484. doi:10.1351/pac-rep-10-01-05
- ^ Battelle, 2015. Attenuation Pathways for Munitions Constituents in Soils and Groundwater, NAVFAC Technical Report - TR-NAVFAC-EXWC-EV-1503 Report pdf
- ^ Mahendra, S. and Alvarez-Cohen, L., 2006. Kinetics of 1, 4-dioxane biodegradation by monooxygenase-expressing bacteria. Environmental Science & Technology, 40(17), 5435-5442. doi:10.1021/es060714v
- ^ Steffan, R.J., McClay, K.R., Masuda, H. and Zylstra, G.J., 2007. ER-1422: Biodegradation of 1, 4-Dioxane No. CU-1422. Shaw Environmental Inc Lawrenceville NJ. ER-1422
- ^ Adamson, D.T., Anderson, R.H., Mahendra, S. and Newell, C.J., 2015. Evidence of 1, 4-dioxane attenuation at groundwater sites contaminated with chlorinated solvents and 1, 4-dioxane. Environmental Science & Technology, 49(11), 6510-6518. doi:10.1021/acs.est.5b00964
- ^ 10.0 10.1 10.2 10.3 Wiedemeier, T.H., Newell, C.J., Rifai, H.S., and Wilson, J.T., 1999. Natural attenuation of fuels and chlorinated solvents in the subsurface. John Wiley & Sons. doi:1 0.1002/9780470172964
- ^ 11.0 11.1 11.2 11.3 Lawrence, S.J., 2006. Description, Properties, and Degradation of Selected Volatile Organic Compounds Detected in Ground Water--A Review of Selected Literature No. 2006-1338. Report pdf
- ^ 12.0 12.1 Maness, A.D., Bowman, K.S., Yan, J., Rainey, F.A. and Moe, W.M., 2012. Dehalogenimonas spp. can reductively dehalogenate high concentrations of 1, 2-dichloroethane, 1, 2-dichloropropane, and 1, 1, 2-trichloroethane. AMB Express, 2(1), 54. doi:10.1186/2191-0855-2-54
- ^ 13.0 13.1 Spain, J.C. ed., 1995. Biodegradation of nitroaromatic compounds. Environmental Science Research, 49. doi 10.1007/978-1-4757-9447-2
- ^ Field, J.A. and Sierra-Alvarez, R., 2008. Microbial degradation of chlorinated benzenes. Biodegradation, 19(4), 463-480. doi:10.1007/s10532-007-9155-1
- ^ Adrian, L. and Görisch, H., 2002. Microbial transformation of chlorinated benzenes under anaerobic conditions. Research in Microbiology, 153(3), 131-137. doi:10.1016/s0923-2508(02)01298-6
- ^ Prenafeta-Boldú, F.X., Vervoort, J., Grotenhuis, J.T.C. and Van Groenestijn, J.W., 2002. Substrate interactions during the biodegradation of benzene, toluene, ethylbenzene, and xylene (BTEX) hydrocarbons by the fungus Cladophialophora sp. strain T1. Applied and Environmental Microbiology, 68(6), 2660-2665. doi: 10.1128/aem.68.6.2660-2665.2002
- ^ Zeeb, P. and Wiedemeier, T.H., 2007. Technical protocol for evaluating the natural attenuation of MTBE. API Publication 4761. Report pdf
- ^ USEPA, 2007. Monitored Natural Attenuation of Tertiary Butyl Alcohol (TBA) in Ground Water at Gasoline Spill Sites. EPA 600-R-07-100. Report pdf
- ^ Schlötelburg, C., Von Wintzingerode, F., Hauck, R., Hegemann, W. and Göbel, U.B., 2000. Bacteria of an anaerobic 1, 2-dichloropropane-dechlorinating mixed culture are phylogenetically related to those of other anaerobic dechlorinating consortia. International Journal of Systematic and Evolutionary Microbiology, 50(4), 1505-1511. doi:10.1099/00207713-50-4-1505
- ^ Katsoyiannis, I.A. and Zouboulis, A.I., 2004. Application of biological processes for the removal of arsenic from groundwaters. Water Research, 38(1), 17-26. doi 10.1016/j.watres.2003.09.011
- ^ Keimowitz, A.R., Mailloux, B.J., Cole, P., Stute, M., Simpson, H.J. and Chillrud, S.N., 2007. Laboratory investigations of enhanced sulfate reduction as a groundwater arsenic remediation strategy. Environmental Science & Technology, 41(19), 6718-6724. doi 10.1021/es061957q
- ^ Onstott, T.C., Chan, E., Polizzotto, M.L., Lanzon, J. and DeFlaun, M.F., 2011. Precipitation of arsenic under sulfate reducing conditions and subsequent leaching under aerobic conditions. Applied Geochemistry, 26(3), 269-285. doi 10.1016/j.apgeochem.2010.11.027
- ^ Jeyasingh, J., Somasundaram, V., Philip, L. and Bhallamudi, S.M., 2011. Pilot scale studies on the remediation of chromium contaminated aquifer using bio-barrier and reactive zone technologies. Chemical Engineering Journal, 167(1), 206-214. doi 10.1016/j.cej.2010.12.024
- ^ Freese, K., Miller, R., J Cutright, T. and Senko, J., 2014. Review of Chromite Ore Processing Residue (COPR): Past Practices, Environmental Impact and Potential Remediation Methods. Current Environmental Engineering, 1(2), 82-90. doi 10.2174/221271780102141117101551
- ^ Hunter, W.J. and Kuykendall, L.D., 2005. Removing selenite from groundwater with an in situ biobarrier: laboratory studies. Current Microbiology, 50(3), 145-150. doi 10.1007/s00284-004-4418-0
- ^ Anderson, R.T., Vrionis, H.A., Ortiz-Bernad, I., Resch, C.T., Long, P.E., Dayvault, R., Karp, K., Marutzky, S., Metzler, D.R., Peacock, A., White, D.C., , Lowe, M., Lovley, D.R., 2003. Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Applied and Environmental Microbiology, 69(10), 5884-5891. doi 10.1128/aem.69.10.5884-5891.2003
- ^ Finneran, K.T., Anderson, R.T., Nevin, K.P. and Lovley, D.R., 2002. Potential for bioremediation of uranium-contaminated aquifers with microbial U(VI) reduction. Soil and Sediment Contamination: An International Journal, 11(3), 339-357. doi 10.1080/20025891106781
- ^ 28.0 28.1 Dvorak, D.H., Hedin, R.S., Edenborn, H.M. and McIntire, P.E., 1992. Treatment of metal‐contaminated water using bacterial sulfate reduction: Results from pilot‐scale reactors. Biotechnology and Bioengineering, 40(5), 609-616. doi 10.1002/bit.260400508
- ^ 29.0 29.1 Drever, J.I., The Geochemistry of Natural Waters: Surface and Groundwater Environments. Prentice-Hall, Inc., ISBN 0132727900.
- ^ Blowes, D.W., Ptacek, C.J., Benner, S.G., McRae, C.W., Bennett, T.A. and Puls, R.W., 2000. Treatment of inorganic contaminants using permeable reactive barriers. Journal of Contaminant Hydrology, 45(1), 123-137.
- ^ Hashim, M.A., Mukhopadhyay, S., Sahu, J.N. and Sengupta, B., 2011. Remediation technologies for heavy metal contaminated groundwater. Journal of Environmental Management, 92(10), 2355-2388.
- ^ 32.0 32.1 Stroo, H.F. and Ward, C.H. eds., 2010. In situ remediation of chlorinated solvent plumes. Springer Science & Business Media. doi 10.1007/978-1-4419-1401-9
- ^ Air Force Center for Environmental Excellence, Naval Facilities Engineering Service Center, and ESTCP, 2004. Principles and Practices of Enhanced Anaerobic Bioremediation of Chlorinated Solvents. ADA511850.
- ^ 34.0 34.1 34.2 ITRC, 2008. In Situ Bioremediation of Chlorinated Ethene: DNAPL Source Zones. June, 2008.
- ^ USEPA, 2010. Green Remediation Best Management Practices: Bioremediation. EPA 542-F-10-006. Report pdf
- ^ Cope, N. and Hughes, J.B., 2001. Biologically-enhanced removal of PCE from NAPL source zones. Environmental Science & Technology, 35(10), 2014-2021. doi 10.1021/es0017357
- ^ Yang, Y. and McCarty, P.L., 2002. Comparison between donor substrates for biologically enhanced tetrachloroethene DNAPL dissolution. Environmental Science & Technology, 36(15), 3400-3404. doi 10.1021/es011408e
- ^ CL:AIRE, 2010. Results of Laboratory Column Studies to Determine the Potential for Bioremediation of Chlorinated Solvent DNAPL Source Areas. SABRE Bulletin 3.
- ^ Moretti, L., 2005. In situ bioremediation of DNAPL Source Zones. US Environmental Protection Agency, Office of Solid Waste and Emergency Response, Technology Innovation and Field Services Division.
- ^ Wei, N. and Finneran, K.T., 2011. Influence of ferric iron on complete dechlorination of trichloroethylene (TCE) to ethene: Fe(III) reduction does not always inhibit complete dechlorination. Environmental Science & Technology, 45(17), 7422-7430. doi 10.1021/es201501a
- ^ Vogel, T.M., Criddle, C.S. and McCarty, P.L., 1987. ES&T critical reviews: transformations of halogenated aliphatic compounds. Environmental Science & Technology, 21(8), 722-736. doi:10.1021/es00162a001
- ^ Bradley, P.M. and Chapelle, F.H., 1996. Anaerobic mineralization of vinyl chloride in Fe (III)-reducing, aquifer sediments. Environmental Science & Technology, 30(6), 2084-2086. doi 10.1021/es950926k
- ^ Parsons, 2004. Principles and Practices of Enhanced Anaerobic Bioremediation of Chlorinated Solvents. AFCEE, NFEC, ESTCP. Report pdf
- ^ Major, D.W., McMaster, M.L., Cox, E.E., Edwards, E.A., Dworatzek, S.M., Hendrickson, E.R., Starr, M.G., Payne, J.A. and Buonamici, L.W., 2002. Field demonstration of successful bioaugmentation to achieve dechlorination of tetrachloroethene to ethene. Environmental Science & Technology, 36(23), 5106-5116. doi 10.1021/es0255711
- ^ Hendrickson, E.R., Payne, J.A., Young, R.M., Starr, M.G., Perry, M.P., Fahnestock, S., Ellis, D.E. and Ebersole, R.C., 2002. Molecular analysis of Dehalococcoides 16S ribosomal DNA from chloroethene-contaminated sites throughout North America and Europe. Applied and Environmental Microbiology, 68(2), 485-495. doi 10.1128/aem.68.2.485-495.2002
- ^ Lendvay, J.M., Löffler, F.E., Dollhopf, M., Aiello, M.R., Daniels, G., Fathepure, B.Z., Gebhard, M., Heine, R., Helton, R., Shi, J., Krajmalnik-Brown, R., Major Jr., C.L., Barcelona, M.J., Petrovskis, E., Hickey, R., Tiedje, J.M., Adriaens, P., 2003. Bioreactive barriers: a comparison of bioaugmentation and biostimulation for chlorinated solvent remediation. Environmental Science & Technology, 37(7), 1422-1431. doi 10.1021/es025985u
- ^ USEPA 2016. Anaerobic Bioremediation (Direct) Application.
- ^ 48.0 48.1 ITRC, 2007. In Situ Bioremediation of Chlorinated Ethene DNAPL Source Zones: Case Studies, BioDNAPL-2, 173 pp, 2007.Report pdf
- ^ ESTCP, 2008. Biodegradation of Dense Non-Aqueous Phase Liquids (DNAPLs) through Bioaugmentation of Source Areas - Dover National Test Site, Dover, Delaware: ESTCP Cost and Performance Report. ESTCP Project ER-0008
- ^ Lieberman, M.T. and Borden, R.C., 2009. Edible Oil Barriers for Treatment of Chlorinated Solvent Contaminated Groundwater. Solutions Industrial and Environmental Services Raleigh, NC. Report pdf
- ^ 51.0 51.1 Cox, E. and Krug, T., 2012. Comparative Demonstration of Active and Semi-Passive In Situ Bioremediation Approaches for Perchlorate Impacted Groundwater: Active In Situ Bioremediation Demonstration (Aerojet Facility) ESTCP Project ER-200219. ER-200219
- ^ 52.0 52.1 Henry, B., 2010. Loading Rate and Impacts of Substrate Delivery for Enhanced Anaerobic Bioremediation. ESTCP Project ER-200627, 90 pgs. ER-200627
- ^ Robinson, C., Barry, D.A., McCarty, P.L., Gerhard, J.I. and Kouznetsova, I., 2009. pH control for enhanced reductive bioremediation of chlorinated solvent source zones. Science of the Total Environment, 407(16), 4560-4573. doi 10.1016/j.scitotenv.2009.03.029
- ^ Borden, R.C., Cha, K.Y., Simpkin, T. and Lieberman, M.T., 2012. Development of a Design Tool for Planning Aqueous Amendment Injection Systems Soluble Substrate Design Tool (No. ER-200626). North Carolina State Univ. at Raleigh. Report pdf
See Also
- Bioremediation
- In Situ and Ex Situ Biodegradation Technologies for Remediation of Contaminated Sites
- Bioaugmentation For Remediation of Chlorinated Solvents
- Principles and Practices of Enhanced Anaerobic Bioremediation of Chlorinated Solvents
- Bioremediation of DNAPLs
- In Situ Bioremediation
- Using Bioremediation in Dense Non-Aqueous Phase Liquid Source Zones
- Design Considerations for Enhanced Reductive Dechlorination
- Novel Approach for Stimulating Reductive Dechlorination of Solvents
- Foam Delivery of Hydrogen for Enhanced Aquifer Contacting and Anaerobic Bioremediation of Chlorinated Solvents
- Development of Permeable Reactive Barriers Using Edible Oils
- Low-Volume Pulsed Biosparging of Hydrogen for Bioremediation of Chlorinated Solvent Plumes
- Demonstration of Bioaugmentation at Kelly Air Force Base, Texas
- Reductive Anaerobic Biological In Situ Treatment Technology (RABITT) Treatability Testing
- Molasses-Induced Reactive Zones to Treat Chlorinated Hydrocarbons
- Evaluation of Performance and Costs Associated with Anaerobic Dechlorination
- Edible Oil Barriers for Treatment of Chlorinated Solvent- and Perchlorate-Contaminated Groundwater
- Field Comparison of Biofouling Control Measures for In Situ Bioremediation of Groundwater
- Reductions in DNAPL Longevity Through Biological Flux Enhancement
- A Low-Cost, Passive Approach for Bacterial Growth and Distribution for Large-Scale Implementation of Bioaugmentation
- Bioaugmentation for Groundwater Remediation
- Development of Design Tools for Planning Aqueous Amendment Injection Systems
- Loading Rates and Impacts of Substrate Delivery for Enhanced Anaerobic Bioremediation
- Improving Effectiveness of Bioremediation at DNAPL Source Zone Sites Applying Partitioning Electron Donors
- In Situ Bioremediation of Chlorinated Solvents Source Areas with Enhanced Mass Transfer
- Enhanced Attenuation of Unsaturated Chlorinated Solvent Source Zones Using Direct Hydrogen Delivery