Difference between revisions of "Chlorinated Solvents"
m (1 revision imported) |
m (1 revision imported) |
(No difference)
| |
Revision as of 20:15, 27 April 2018
Chlorinated solvents, including chlorinated volatile organic compounds (CVOC or CVOCs), are chemical compounds containing chlorine that have been widely used in various industries. They are divided in three groups (methanes, ethanes, ethenes) based on their structures, and include common groundwater contaminants such as carbon tetrachloride (CT), perchloroethene (PCE), trichloroethene (TCE), and vinyl chloride (VC). Chlorinated solvents tend to be colorless liquids at room temperatures, heavier than water, volatile, sparingly soluble, and moderately hydrophobic.
Related Article(s):
- Soil & Groundwater Contaminants
- Monitored Natural Attenuation (MNA) of Chlorinated Solvents
- Biological Reductive Processes
CONTRIBUTOR(S): Dr. Bilgen Yuncu, P.E. and M. Tony Lieberman
Key Resource(s):
Introduction
Chlorinated solvents are a large family of organic solvents that contain chlorine atoms in their molecular structure. They were first produced in Germany in the 1800s, and widespread use in the United States (U.S.) began after World War II. In the period of 1940-1980, the U.S. produced about 2 billion pounds of chlorinated solvents each year[2]. Chlorinated solvents, including carbon tetrachloride (CT), 1,1,1-trichloroethane (TCA), perchloroethene or tetrachloroethene (PCE) and trichloroethene (TCE) have been among the most widely used cleaning and degreasing solvents in the U.S[3]. They also have been used in a wide variety of other purposes such as adhesives, chemical intermediates, clothes, pharmaceuticals, pesticides, and textile processing.
Physical & Chemical Properties
Chlorinated solvents are organic compounds generally constructed of a simple hydrocarbon chain (typically one to three carbon atoms in length). They can be divided into three categories based on their structural characteristics: chlorinated methanes, chlorinated ethanes and chlorinated ethenes.
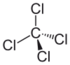
Chlorinated methanes represent the most structurally simple solvent class and consist of a single carbon center (known as a methyl carbon) to which as many as four chlorine atoms are bonded. From the perspective of groundwater contamination, perhaps the most well-known chlorinated methanes are carbon tetrachloride (CT) or tetrachloromethane, trichloromethane (commonly known as chloroform [CF]), dichloromethane (DCM), or methylene chloride (MC) and chloromethane (CM), or methyl chloride.
Chlorinated ethanes consist of two carbon centers joined by a single covalent bond. The most frequently encountered groundwater pollutants of this class include 1,1,1-trichloroethane (1,1,1-TCA) and 1,2-dichloroethane.
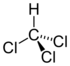
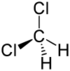
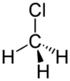
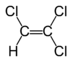
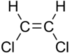
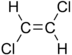
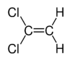
Chlorinated ethenes (also referred to as chlorinated ethylenes) also possess two carbon centers, but unlike chlorinated ethanes, these carbon atoms are joined by a carbon-carbon double bond. Chlorinated ethenes that are important groundwater contaminants include tetrachloroethene, or perchloroethene (PCE), trichloroethene (TCE), dichloroethene (DCE)) (DCE, mainly two geometric isomers cis-1,2-dichloroethene and trans-1,2-dichloroethene), and vinyl chloride (VC).
Nomenclature and structure of selected compounds from each solvent class as well as some physical and chemical properties of most widely used chlorinated solvents are listed in Table 1.
| IUPAC Name | Common Name | Acronym | Molecular Formula | Chemical Structure | Formula Weight | Density (ρ)(g/mL) | Aqueous Solubility (mg/L) | Vapor Pressure (ρ0)(kPa) | Henry's Law Constanta | Log Kow | MCLb (μg/L) |
| Chlorinated Methanes | |||||||||||
| tetrachloromethane | carbon tetrachloride | CT | CCl4 | 153.8 | 1.59 | 800 | 20.5 | 28.9 | 2.64 | 0.005 | |
| trichloromethane | chloroform | CF | CHCl3 | 119.4 | 1.49 | 8,200 | 26.2 | 3.8 | 1.97 | 0.080c | |
| dichloromethane | methylene chloride | DCM | CH2Cl2 | 84.9 | 1.33 | 13,200 | 55.3 | 1.7 | 1.25 | 0.005 | |
| chloromethane | methyl chloride | CM | CH3Cl | 50.5 | 0.92 | 5,235 | 570 | 9.6 | 0.91 | NRd | |
| Chlorinated Ethanes | |||||||||||
| hexachloroethane | perchloroethane | HCA | C2Cl6 | 236.7 | 2.09 | 50 | 0.05e | - | 3.93 | NR | |
| pentachloroethane | - | PCA | C2HCl5 | 202.3 | 1.68 | 500 | 0.6 | 2.5 | 2.89 | NR | |
| 1,1,1,2-tetrachloroethane | - | 1,1,1,2-TeCA | C2H2Cl4 | 167.9 | 1.54 | 1,100 | 1.6 | 2.4 | - | NR | |
| 1,1,2,2-tetrachloroethane | - | 1,1,2,2-TeCA | C2H2Cl4 | 167.9 | 1.60 | 2,962 | 0.8 | 0.44 | 2.39 | NR | |
| 1,1,2-trichloroethane | - | 1,1,2-TCA | C2H3Cl3 | 133.4 | 1.44 | 4,394 | 3.22 | 0.96 | 2.38 | 0.005 | |
| 1,1,1-trichloroethane | methyl chloroform | 1,1,1-TCA | C2H3Cl3 | 133.4 | 1.35 | 1,495 | 16.5 | 14.5 | 2.49 | 0.20 | |
| 1,2-dichloroethane | - | 1,2-DCA | C2H4Cl2 | 99.0 | 1.25 | 8,606 | 10.5 | 1.2 | 1.48 | 0.005 | |
| 1,1-dichloroethane | - | 1,1-DCA | C2H4Cl2 | 99.0 | 1.17 | 4,676 | 30.3 | 6.2 | 1.79 | NR | |
| chloroethane | - | CA | C2H5Cl | 64.5 | 0.92 | 5,700 | 16.0 | 1.8 | 1.43 | NR | |
| Chlorinated Ethenes | |||||||||||
| tetrachloroethene | perchloroethene | PCE | C2Cl4 | 165.8 | 1.63 | 150 | 2.4 | 26.3 | 2.88 | 0.005 | |
| trichloroethene | - | TCE | C2HCl3 | 131.4 | 1.46 | 1,100 | 9.9 | 11.7 | 2.53 | 0.005 | |
| cis-1,2-dichloroethene | cis-dichloroethene | cis-DCE | C2H2Cl2 | 96.9 | 1.28 | 3,500 | 27.1 | 7.4 | 1.86 | 0.07 | |
| trans-1,2-dichloroethene | trans-dichloroethene | trans-DCE | C2H2Cl2 | 96.9 | 1.26 | 6,260 | 44.4 | 6.8 | 1.93 | 0.1 | |
| 1,1-dichloroethene | vinylidene chloride | 1,1-DCE | C2H2Cl2 | 96.9 | 1.22 | 3,344 | 80.5 | 23.0 | 2.13 | 0.007 | |
| chloroethene | vinyl chloride | VC | C2H3Cl | 62.5 | 0.91 | 2,763 | 355 | 79.2 | 1.38 | 0.002 | |
| Notes:
atm = atmosphere; g = gram; Kow = octanol/water partitioning coefficient; Koc -- soil organic carbon/water partitioning coefficient; L = liter; MCL = maximum contaminant level; mg = milligram; mL = milliliter; mol = mole.
bSource: http://water.epa.gov/drink/contaminants/#List cMCL for total trihalomethanes is defined as the summed concentration of chloroform, bromoform (CHBr3),bromodichloromethane (CHBrCl2), and dibromochloromethane (CHBr2Cl). http://water.epa.gov/drink/contaminants/basicinformation/disinfectionbyproducts.cfm dNR : Not regulated. eReported vapor pressure for solid-phase hexachloroethane. | |||||||||||
Chlorinated solvents and many of their transformation products are colorless liquids at room temperature. They are heavier than water with densities greater than 1 gram per cubic centimeter (g/cm3) which means they can penetrate deeply into an aquifer. They are relatively volatile compounds with relatively high Henry’s Law constants(KH), a measure of the strength of partitioning from water into air). Generally, when KH for a compound exceeds 0.2 atmosphere/mole fraction (atm/M), they can readily be removed from water by air stripping it. Most chlorinated solvents can be classified as sparingly soluble in water, with aqueous solubilities generally on the order of 10s to 100s of mg/L. As the number of chlorine atoms on a compound increases, the solubility decreases. Because of their relatively low solubilities, chlorinated solvents dissolve slowly in groundwater. Another consequence of their limited solubility is their tendency to occur in the subsurface as a separate immiscible liquid phase which, because of its density compared to water, tends to sink in groundwater. Under these conditions, these are referred to as dense non-aqueous phase liquid (DNAPL). Although chlorinated solvents are not very soluble in water, their solubility is typically orders of magnitude greater than their established drinking water standards.
Chlorinated solvents can be considered moderately hydrophobic which can be determined by their octanol-water partition coefficients (Kow, a measure of the tendency of a substance to prefer an organic or oily phase rather than an aqueous phase). Log Kow values less than 3 indicate that the compound does not sorb strongly to aquifer solids, but can be removed readily by activated carbon. On the other hand, compounds with log Kow less than 2, such as VC, generally are not removed well by activated carbon either[1].
References
- ^ 1.0 1.1 1.2 Cwiertny, D.M., Scherer, M.M., 2010. Chlorinated solvent chemistry: structures, nomenclature and properties. In In situ remediation of chlorinated solvent plumes. Springer New York. pgs. 29-37. doi:10.1007/978-1-4419-1401-9_2
- ^ Pankow, J.F., Cherry, J.A., 1996. Dense Chlorinated Solvents and Other DNAPLs in Groundwater, Waterloo Press, Portland, OR. ISBN 0964801418
- ^ Doherty, R.E., 2000. A history of the production and use of carbon tetrachloride, tetrachloroethylene, trichloroethylene and 1, 1, 1-trichloroethane in the United States: Part 1--historical background; carbon tetrachloride and tetrachloroethylene. Environmental Forensics, 1(2), 69-81. doi:10.1006/enfo.2000.0010