Metal(loid)s - Small Arms Ranges
Metals and metalloids are deposited into small arms range soils from bullets, casings, and primers. The mobility potential depends on the individual metal(loid), its speciation, local pH, redox conditions and soil characteristics. This article discusses the speciation, mobility, and toxicity of metal(loid)s relevant to small arms ranges and briefly discusses management strategies to mitigate risk.
Related Article(s):
- Metals and Metalloids - Mobility in Groundwater
- Monitored Natural Attenuation (MNA) of Metal and Metalloids
Contributor(s): Dr. Amanda Barker
Key Resource(s):
- Army Small Arms Training Range Environmental Best Practices (BMPs) Manual
- Management of the Environmental Impact of Shooting Ranges, the Finnish Environment
Introduction
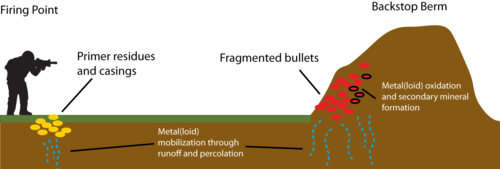
Military small arms training uses bullets made of metals and metalloids which can include lead, antimony, copper, nickel, zinc, chromium, tungsten, mercury, and arsenic. Some of these metal(loid)s are also key ingredients of small arms ammunition primers used to initiate bullet firing. Unlike organic compounds found in propellants and explosives, metal(loid)s do not degrade in the environment. As such, the pervasive use of metal(loid)s on military training grounds and shooting ranges has resulted in contamination of soils, sediments, surface water, and groundwater both local to specific sites and off-site. A simplified schematic showing potential pathways of metal(loid) release on shooting ranges is shown in Figure 1. Upon exposure to the atmosphere, initially zero-valent metal(loid)s are oxidized, form secondary mineral phases, and subsequently release oxidized species into soil solution. The formation of a weathering crust on the surface of fired bullets is shown in Figures 2 and 3. Mobilization of metal(loid)s in pore water and soil solution is controlled by a variety of factors, including pH, oxidation-reduction (redox) environment, presence of colloids and/or soil organic matter (SOM), soil type and hydraulic characteristics, temperature, and water infiltration[1][2][3][4].

Metal(loid)s
Lead (Pb)
Lead (Pb) and antimony alloy bullet cores are commonly used in small arms ammunition to provide the high density needed to penetrate surfaces. Pb is estimated to comprise approximately 90-99% of the bullet core with steel, copper, and zinc making up the majority of the remaining mass for the slug and casing[1][4]. Pb is also used in the compounds lead styphnate and lead azide as primers for ammunition. As a result, Pb can be found on training ranges in soils/structures where the bullet fragments and also at the firing point[5]. Concentrations of Pb in soils at shooting ranges can vary widely depending on the number of rounds fired at the site, but concentrations often range from 55 to 80,000 mg/kg[1][6][7][8]. The mobility of Pb is highly dependent on local pH. Acidic conditions contribute to the mobilization of Pb species in the environment whereas basic conditions tend to promote precipitation and sorption reactions[9][10]. The formation of secondary mineral phases is a common observation, including hydrocerussite [Pb3(CO3)2(OH)2], cerussite [PbCO3], litharge and massicot [PbO polymorphs], but Pb also binds with sulfur and phosphorus depending on mineralogical conditions at a given site[9][1][11][12]. Additionally, Pb has been known to bind with soil organic matter, clay minerals, and iron/aluminum/manganese - oxides[13][14]. Pb is found in the near-surface environment in one oxidation state, Pb2+. Its toxicity is manifested primarily by effects to the central nervous system and brain function, but it can also cause damage to reproductive processes, auditory function, and vision[15].
Antimony
Antimony (Sb) is a hard, toxic, metalloid that is added to Pb in molten form to make the Pb-alloyed core of a bullet. Sb is thought to behave similarly in the environment to its group VB neighbor Arsenic (As), and, like As, it is toxic and a suspected carcinogen[16]. Sb is added to bullets as a hardening agent because Pb is soft and would not have the same impact capacity without it[2][7]. Bullet cores typically contain no more than 2% Sb and, correspondingly, concentrations in soils are typically lower than for Pb. Sb is also often found in the primers of small arms ammunition as Sb sulfide. Although Sb is present in bullets approximately two orders of magnitude less than Pb, in some aqueous systems Sb has been found to be more mobile than Pb[17][18][19]. However, in more acidic soils Pb often exceeds measured Sb aqueous concentrations due to the high solubility of Pb at low pH values[20]. The mobility of Sb on ranges is highly dependent on its speciation, which in near-surface environments is Sb(III) or Sb(V). The trivalent form has been shown to be less mobile than the pentavalent form, but the trivalent form is considerably more toxic, particularly Sb trioxide[2][21]. Sb(V) is more mobile at higher pHs[22], while Sb(III) in solution is independent of pH[17]. Certain environmental factors influence Sb speciation, including pH, redox potential, biological activity, and co-contamination[23][21][24][19]. Sb oxidation is rapid[24], forming secondary products both in soils and within the weathering crust surrounding bullets[3]. Iron (Fe) is a natural sink for Sb, forming primarily inner sphere sorption complexes (both corner and edge-sharing) with Fe(III) oxides found in soils[2][3][25].
Copper
Copper (Cu) is an essential nutrient for the body and has a relatively high maximum contaminant level (MCL) in drinking water set by the EPA (1.3 mg/kg) compared to other metal(loid)s that comprise bullets. However, toxicity from elevated Cu concentrations can arise and cause gastrointestinal distress (short-term) and liver and/or kidney damage (long-term)[26]. The outer casing and jacket of a bullet is comprised primarily of Cu. When a bullet is fired, the outer Cu casing remains at the firing point along with the primer and propellant residues while the Cu jacket impacts soil and either deforms or fragments. New 5.56-mm and 7.62-mm “green” bullets contain solid Cu cores in place of Pb/Sb alloys. Copper concentrations in shooting range soils typically range from 19 to upwards of 6,000 mg/kg[27][8]. Similar to Pb, Cu mobility is highly dependent on pH[28], and it has been shown to easily complex with organic matter[29][30].
Nickel
Nickel (Ni) toxicity is typically a concern to humans due to its prevalence as an allergen and the ability of Ni to interfere with other essential metals in cellular processes[31][32]. Trace amounts of Ni are found in the casing and jacket of a bullet, used to make the alloy with Cu. Ni is a biologically important micronutrient and an essential trace element for plants, microbes, animals, and humans[33][34][35]. Ni concentrations in environmental systems tend to be conservative and uniformly distributed[36]. In shooting ranges, typical concentrations of Ni are lower compared to the other metal(loid)s like Pb, Sb, Cu, and Zn[37]. Ni tends to be relatively immobile in soil solution under neutral and alkaline conditions, but can become highly mobilized under acidic conditions (similar to the other cationic metals; Pb, Cu, and Zn). Similar to other metal(loid)s, key environmental processes that affect Ni cycling in geomedia include adsorption, (co)precipitation, and complexation[38].
Zinc
Zinc (Zn) is an essential micronutrient for sustained existence[34]. Similar to Ni, trace amounts of Zn are found in the casing and jacket of a bullet, used to make the alloy with Cu. Zn has been found to become mobilized at acidic pHs, similar to other cationic metals (Pb, Cu, and Ni) and often associates with clay minerals, soil organic matter, and the roots and shoots of plants, if available [39][40].
Chromium
Chromium (Cr) in the +3 valence state (Cr(III)) is an essential micronutrient, but has been found to be toxic in large doses. Chromium in the +6 valence state (Cr(VI)) is classified as a human carcinogen[41][42]. Depending on the quality of the source of metals used for the production of bullets, Cr may be found as an impurity in steel[43][44][8]. One study on Cr presence at shooting range soils estimated the Cr content of steel to be up to 27%, which is a much higher estimate than other studies have reported[43]. Typically, Cr concentrations in shooting range soils are reported comparable to background concentrations in local soils, indicating minimal environmental impact from training[8]. Cr is primarily found in the near-surface environment as Cr(III) and Cr(VI). In its hexavalent oxidation state Cr is highly mobile, while Cr(III) is more likely to participate in precipitation and sorption reactions, and therefore mobility in aqueous environments and sediments is limited[45].
Tungsten
Exposure to high tungsten (W) concentrations has been linked to altered thyroid function, heart disease, and potentially childhood leukemia and lung cancer[46]. Historically, W and nylon have been used as an alternative for Pb in bullets[46]. The tungsten-nylon bullets were termed ‘green bullets’, but did not stay in production for longer than a decade as subsequent research eventually revealed W to be both toxic and highly mobile[47]. Concentrations of W in shooting range soils where green bullets have been spent ranged from below detection to greater than 2,000 mg/kg[48]. W has been shown to be relatively mobile in the environment, with its mobility dependent on speciation. However, there is evidence to suggest W mobility decreases as soil ages, as long as the source is limited[49]. W metal is not stable in the environment and instead primarily exists as W(VI) and prefers to form a variety of polyatomic anions (like WO42- and H2W12O406-, etc.) and polyoxometalates in solution[50].
Mercury
A compound containing mercury (Hg) (mercury fulminate, Hg(CNO)2) was previously used as a primer for ammunition for approximately 100 years before it was widely replaced by lead azide, silver azide, or lead styphnate which have greater stability[51][5]. Despite the minimal to negligible use in recent history, there is some evidence to suggest that legacy shooting ranges may have persistent Hg contamination in soil[52][53]. Additionally, marine environments near legacy military training grounds may contain elevated concentrations of Hg from the dumping of ammunition after World War II, including the Baltic Sea[54]. Similar to other primer metal(loid)s, Hg in firing range soils should primarily be located near the firing point instead of traveling with the bullet. An old Swiss shooting range was found to have slightly elevated total Hg concentrations above background values (44-315 μg/kg) with maximum values ranging from 906 to 1,517 μg/kg in soils at the firing point, with concentrations decreasing with increasing distance from the firing point[52]. While the concentrations of Hg found at shooting ranges are significantly lower than concentrations reported for Pb and Sb, it still remains a health and environmental hazard due to its harmful effects and toxicity even at low concentrations[53].
Arsenic

Arsenic (As) is a highly toxic and carcinogenic metalloid that affects nearly all organ systems[55]. Arsenic has been found at shooting ranges to a much lesser extent than other metal(loid)s, as it is associated with Pb as an impurity. While As is a well-known contaminant in other systems, at shooting ranges it is often not measured or measured at concentrations close to background concentrations in nearby soils[6][56]. Arsenic exhibits multiple oxidation states in the near-surface environment, but is most often found as arsenite (As(III)) and/or arsenate (As(V)). Major environmental factors that affect As mobility are pH, redox condition, and soil type[57].
Management Practices
Range design is an important factor in containing metal(loid)s and preventing their migration into the subsurface. Target berms may be comprised of a natural hillside or slope, or constructed with off-site source material that is shaped into an artificial barrier (as shown in Figure 4). Ensuring berm construction includes a berm face that is steep enough to prevent bullet skipping, but stable enough to minimize soil erosion, can minimize contaminated sediment losses. Siting the berm with respect to hydrologic pathways to waterbodies, aquifers, and potential ecological receptors is crucial. Additionally, it is important to decide what type of soil will comprise the berm or hillside (i.e. sand, organic-rich material, etc.) and determining soil texture and major mineral and element fractions. These soil composition variables will affect the overall speciation and mobility of the target contaminant species in bullets (Pb, Sb, etc.). Bullet catchers (see Additional Resources) are designed to catch bullets and contain bullet-related metal(loid)s for later collection and disposal. These systems are designed for long-term use on range. The bullet-related metal(loid)s are stored internally in a protective liner to minimize soil and water interactions with the fired bullet rounds.
Additionally, soil amendments such as phosphate and lime can alter soil pH to inhibit mobility of some metals (mainly cationic metals like Pb, Cu, Ni, and Zn), and other amendments such as Fe/Mn hydro(oxides) can facilitate sorption reactions with metal(loid)s, such as Sb and As. Although costly, spent bullets and large bullet fragments can also be removed from soils.
References
- ^ 1.0 1.1 1.2 1.3 Vantelon, D., Lanzirotti, A., Scheinost, A.C. and Kretzschmar, R., 2005. Spatial distribution and speciation of lead around corroding bullets in a shooting range soil studied by micro-X-ray fluorescence and absorption spectroscopy. Environmental Science & Technology, 39(13), pp.4808-4815. doi: 10.1021/es0482740
- ^ 2.0 2.1 2.2 2.3 Scheinost, A.C., Rossberg, A., Vantelon, D., Xifra, I., Kretzschmar, R., Leuz, A.K., Funke, H. and Johnson, C.A., 2006. Quantitative antimony speciation in shooting-range soils by EXAFS spectroscopy. Geochimica et Cosmochimica Acta, 70(13), pp.3299-3312. doi: 10.1016/j.gca.2006.03.020
- ^ 3.0 3.1 3.2 Ackermann, S., Gieré, R., Newville, M. and Majzlan, J., 2009. Antimony sinks in the weathering crust of bullets from Swiss shooting ranges. Science of the Total Environment, 407(5), pp.1669-1682. doi: 10.1016/j.scitotenv.2008.10.059
- ^ 4.0 4.1 Sanderson, P., Qi, F., Seshadri, B., Wijayawardena, A. and Naidu, R., 2018. Contamination, fate and management of metals in shooting range soils—A Review. Current Pollution Reports, 4(2), pp.175-187. doi: 10.1007/s40726-018-0089-5
- ^ 5.0 5.1 Pichtel, J., 2012. Distribution and fate of military explosives and propellants in soil: a review. Applied and Environmental Soil Science, 2012. doi: 10.1155/2012/617236
- ^ 6.0 6.1 Dermatas, D., Cao, X., Tsaneva, V., Shen, G. and Grubb, D.G., 2006. Fate and behavior of metal (loid) contaminants in an organic matter-rich shooting range soil: Implications for remediation. Water, Air, & Soil Pollution: Focus, 6(1-2), pp.143-155. doi: 10.1007/s11267-005-9003-4
- ^ 7.0 7.1 Sanderson, P., Bolan, N., Bowman, M. and Naidu, R., 2010. Distribution and availability of metal contaminants in shooting range soils around Australia. In Proceedings of the 19th World Congress of Soil Science: Soil solutions for a changing world, Brisbane, Australia, 1-6 August 2010. Symposium 3.5. 1 Heavy metal contaminated soils (pp. 65-67). International Union of Soil Sciences (IUSS), c/o Institut für Bodenforschung, Universität für Bodenkultur.
- ^ 8.0 8.1 8.2 8.3 Rodríguez‐Seijo, A., Alfaya, M.C., Andrade, M.L. and Vega, F.A., 2016. Copper, chromium, nickel, lead and zinc levels and pollution degree in firing range soils. Land Degradation & Development, 27(7), pp.1721-1730. doi: 10.1002/ldr.2497
- ^ 9.0 9.1 Cao, X., Ma, L.Q., Chen, M., Hardison, D.W. and Harris, W.G., 2003. Weathering of lead bullets and their environmental effects at outdoor shooting ranges. Journal of Environmental Quality, 32(2), pp.526-534. doi:10.2134/jeq2003.5260
- ^ Knechtenhofer, L.A., Xifra, I.O., Scheinost, A.C., Flühler, H. and Kretzschmar, R., 2003. Fate of heavy metals in a strongly acidic shooting‐range soil: small‐scale metal distribution and its relation to preferential water flow. Journal of Plant Nutrition and Soil Science, 166(1), pp.84-92. doi: 10.1002/jpln.200390017
- ^ Scheetz, C.D. and Rimstidt, J.D., 2009. Dissolution, transport, and fate of lead on a shooting range in the Jefferson National Forest near Blacksburg, VA, USA. Environmental Geology, 58(3), pp.655-665. doi: 10.1007/s00254-008-1540-5
- ^ Li, J., Wang, Q., Oremland, R.S., Kulp, T.R., Rensing, C. and Wang, G., 2016. Microbial antimony biogeochemistry: enzymes, regulation, and related metabolic pathways. Appl. Environ. Microbiol., 82(18), pp.5482-5495. doi: 10.1128/aem.01375-16
- ^ Manceau, A., Boisset, M.C., Sarret, G., Hazemann, J.L., Mench, M., Cambier, P. and Prost, R., 1996. Direct determination of lead speciation in contaminated soils by EXAFS spectroscopy. Environmental Science & Technology, 30(5), pp.1540-1552. doi: 10.1021/es9505154
- ^ Mozafar, A., Ruh, R., Klingel, P., Gamper, H., Egli, S. and Frossard, E., 2002. Effect of heavy metal contaminated shooting range soils on mycorrhizal colonization of roots and metal uptake by leek. Environmental Monitoring and Assessment, 79(2), pp.177-191. doi: 10.1023/A:1020202801163
- ^ Mason B. (1952) Principles of geochemistry, 276 p. New York, John Wiley and Sons. ISBN: 0471575224
- ^ World Health Organization, 2003. Background document for development of WHO Guidelines for Drinking-water Quality. World Health Organization, 20, pp.4-6.
- ^ 17.0 17.1 Johnson, C.A., Moench, H., Wersin, P., Kugler, P. and Wenger, C., 2005. Solubility of antimony and other elements in samples taken from shooting ranges. Journal of Environmental Quality, 34(1), pp.248-254. doi:10.2134/jeq2005.0248
- ^ Sanderson, P., Naidu, R. and Bolan, N., 2015. Effectiveness of chemical amendments for stabilisation of lead and antimony in risk-based land management of soils of shooting ranges. Environmental Science and Pollution Research, 22(12), pp.8942-8956. doi: 10.1007/s11356-013-1918-0
- ^ 19.0 19.1 Doherty, S.J., Tighe, M.K. and Wilson, S.C., 2017. Evaluation of amendments to reduce arsenic and antimony leaching from co-contaminated soils. Chemosphere, 174, pp.208-217. doi: 10.1016/j.chemosphere.2017.01.100
- ^ Okkenhaug, G., Gebhardt, K.A.G., Amstaetter, K., Bue, H.L., Herzel, H., Mariussen, E., Almås, Å.R., Cornelissen, G., Breedveld, G.D., Rasmussen, G. and Mulder, J., 2016. Antimony (Sb) and lead (Pb) in contaminated shooting range soils: Sb and Pb mobility and immobilization by iron-based sorbents, a field study. Journal of Hazardous Materials, 307, pp.336-343. doi: 10.1016/j.jhazmat.2016.01.005
- ^ 21.0 21.1 Wilson, S.C., Lockwood, P.V., Ashley, P.M. and Tighe, M., 2010. The chemistry and behaviour of antimony in the soil environment with comparisons to arsenic: a critical review. Environmental Pollution, 158(5), pp.1169-1181. doi: 10.1016/j.envpol.2009.10.045
- ^ Hu, X., Guo, X., He, M. and Li, S., 2016. pH-dependent release characteristics of antimony and arsenic from typical antimony-bearing ores. Journal of Environmental Sciences, 44, pp.171-179. doi: 10.1016/j.jes.2016.01.003
- ^ Filella, M., Belzile, N. and Chen, Y.W., 2002. Antimony in the environment: a review focused on natural waters: I. Occurrence. Earth-Science Reviews, 57(1), pp.125-176. doi:10.1016/S0012-8252(01)00070-8
- ^ 24.0 24.1 Ilgen, A.G., Majs, F., Barker, A.J., Douglas, T.A. and Trainor, T.P., 2014. Oxidation and mobilization of metallic antimony in aqueous systems with simulated groundwater. Geochimica et Cosmochimica Acta, 132, pp.16-30. doi:10.1016/j.gca.2014.01.019
- ^ Ritchie, V.J., Ilgen, A.G., Mueller, S.H., Trainor, T.P. and Goldfarb, R.J., 2013. Mobility and chemical fate of antimony and arsenic in historic mining environments of the Kantishna Hills district, Denali National Park and Preserve, Alaska. Chemical Geology, 335, pp.172-188. doi: 10.1016/j.chemgeo.2012.10.016
- ^ U. S. Environmental Protection Agency (USEPA), 2009. National Primary Drinking Water Regulations. EPA 816-F-09-004. Report.pdf
- ^ Robinson, B.H., Bischofberger, S., Stoll, A., Schroer, D., Furrer, G., Roulier, S., Gruenwald, A., Attinger, W. and Schulin, R., 2008. Plant uptake of trace elements on a Swiss military shooting range: Uptake pathways and land management implications. Environmental Pollution, 153(3), pp.668-676. doi: 10.1016/j.envpol.2007.08.034
- ^ Sherene, T., 2010. Mobility and transport of heavy metals in a polluted soil environment. In Biological forum - An International Journal (Vol. 2, No. 2, pp. 112-121). Report.pdf
- ^ Huang, J., Huang, R., Jiao, J.J. and Chen, K., 2007. Speciation and mobility of heavy metals in mud in coastal reclamation areas in Shenzhen, China. Environmental Geology, 53(1), pp.221-228. doi: 10.1007/s00254-007-0636-7
- ^ Ashraf, M.A., Maah, M.J. and Yusoff, I., 2012. Chemical speciation and potential mobility of heavy metals in the soil of former tin mining catchment. The Scientific World Journal, 2012. ISBN: 0471575224
- ^ Coogan, T.P., Latta, D.M., Snow, E.T., Costa, M. and Lawrence, A., 1989. Toxicity and carcinogenicity of nickel compounds. CRC Critical reviews in toxicology, 19(4), pp.341-384. doi: 10.3109/10408448909029327
- ^ Sharma, A.D., 2013. Low nickel diet in dermatology. Indian Journal of Dermatology, 58(3), p.240. doi: 10.4103/0019-5154.110846
- ^ Mason B. (1952) Principles of geochemistry, 276 p. New York, John Wiley and Sons. ISBN: 0471575224
- ^ 34.0 34.1 Coleman D.C., Crossley Jr. D.A. and Hendrix P.F., 2004. Fundamentals of Soil Ecology, 2nd ed. Elsevier Inc. New York City, NY. ISBN: 9780121797263/e: 9780080472812
- ^ Nieminen, T.M., Ukonmaanaho, L., Rausch, N. and Shotyk, W., 2007. Biogeochemistry of nickel and its release into the environment. Metal Ions in Life Sciences, nickel and its surprising impact in nature. 2nd Volume. Chichester: John Wiley & Sons, pp.1-30. ISSN: 1559-0836.
- ^ Iyaka, Y.A., 2011. Nickel in soils: a review of its distribution and impacts. Scientific Research and Essays, 6(33), pp.6774-6777. Report.pdf
- ^ Mariussen, E., Johnsen, I.V. and Strømseng, A.E., 2017. Distribution and mobility of lead (Pb), copper (Cu), zinc (Zn), and antimony (Sb) from ammunition residues on shooting ranges for small arms located on mires. Environmental Science and Pollution Research, 24(11), pp.10182-10196. doi: 10.1007/s11356-017-8647-8
- ^ Harasim, P. and Filipek, T., 2015. Nickel in the environment. Journal of Elementology, 20(2). doi: 10.5601/jelem.2014.19.3.651
- ^ Haslett, B.S., Reid, R.J. and Rengel, Z., 2001. Zinc mobility in wheat: uptake and distribution of zinc applied to leaves or roots. Annals of Botany, 87(3), pp.379-386. doi: 10.1006/anbo.2000.1349
- ^ Rutkowska, B., Szulc, W., Bomze, K., Gozdowski, D. and Spychaj-Fabisiak, E., 2015. Soil factors affecting solubility and mobility of zinc in contaminated soils. International Journal of Environmental Science and Technology, 12(5), pp.1687-1694. doi: 10.1007/s13762-014-0546-7
- ^ Wilbur, S.B., 1998. Toxicological profile for chromium. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry.
- ^ U.S. Environmental Protection Agency (USEPA), 1999. Integrated Risk Information System (IRIS) on chromium VI. National Center for Environmental Assessment. Office of Research and Development, Washington.
- ^ 43.0 43.1 Levonmäki, M. and Kairesalo, T., 2002. Do steel shots raise a chromium problem on shooting range areas. ISSF News, 2, pp.9-10.
- ^ Sorvari, J., Antikainen, R. and Pyy, O., 2006. Environmental contamination at Finnish shooting ranges—the scope of the problem and management options. Science of the Total Environment, 366(1), pp.21-31. doi: 10.1016/j.scitotenv.2005.12.019
- ^ Gorny, J., Billon, G., Noiriel, C., Dumoulin, D., Lesven, L. and Madé, B., 2016. Chromium behavior in aquatic environments: a review. Environmental Reviews, 24(4), pp.503-516. doi: 10.1139/er-2016-0012
- ^ 46.0 46.1 Datta, S., Vero, S.E., Hettiarachchi, G.M. and Johannesson, K., 2017. Tungsten contamination of soils and sediments: current state of science. Current Pollution Reports, 3(1), pp.55-64. doi: 0.1007/s40726-016-0046-0
- ^ Agency for Toxic Substances and Disease Registry (ATSDR), 2005. Toxicological Profile for Tungsten. U.S. Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA.
- ^ Clausen, J.L. and Korte, N., 2009. Environmental fate of tungsten from military use. Science of the total environment, 407(8), pp.2887-2893. doi: 10.1016/j.scitotenv.2009.01.029
- ^ Bednar, A.J., Jones, W.T., Boyd, R.E., Ringelberg, D.B. and Larson, S.L., 2008. Geochemical parameters influencing tungsten mobility in soils. Journal of environmental quality, 37(1), pp.229-233. doi:10.2134/jeq2007.0305
- ^ Bostick, B.C., Sun, J., Landis, J.D. and Clausen, J.L., 2018. Tungsten Speciation and Solubility in Munitions-Impacted Soils. Environmental science & technology, 52(3), pp.1045-1053. doi: 10.1021/acs.est.7b05406
- ^ Beck, W., Evers, J., Göbel, M., Oehlinger, G. and Klapötke, T.M., 2007. The crystal and molecular structure of mercury fulminate (Knallquecksilber). Zeitschrift für anorganische und allgemeine Chemie, 633(9), pp.1417-1422. doi: 10.1002/zaac.200700176
- ^ 52.0 52.1 Stauffer, M., Pignolet, A. and Alvarado, J.C., 2017. Persistent mercury contamination in shooting range soils: the legacy from former primers. Bulletin of environmental contamination and toxicology, 98(1), pp.14-21. doi: 0.1007/s00128-016-1976-3
- ^ 53.0 53.1 Bigham, G.N., Murray, K.J., Masue‐Slowey, Y. and Henry, E.A., 2017. Biogeochemical controls on methylmercury in soils and sediments: Implications for site management. Integrated environmental assessment and management, 13(2), pp.249-263. doi: 10.1002/ieam.1822
- ^ Gębka, K., Bełdowski, J. and Bełdowska, M., 2016. The impact of military activities on the concentration of mercury in soils of military training grounds and marine sediments. Environmental Science and Pollution Research, 23(22), pp.23103-23113. doi: 10.1007/s11356-016-7436-0
- ^ Ratnaike, R.N., 2003. Acute and chronic arsenic toxicity. Postgraduate medical journal, 79(933), pp.391-396. Report.pdf
- ^ Bannon, D.I., Drexler, J.W., Fent, G.M., Casteel, S.W., Hunter, P.J., Brattin, W.J. and Major, M.A., 2009. Evaluation of small arms range soils for metal contamination and lead bioavailability. Environmental science & technology, 43(24), pp.9071-9076. doi: 10.1021/es901834h
- ^ Bissen, M. and Frimmel, F.H., 2003. Arsenic - a review. Part I: occurrence, toxicity, speciation, mobility. Acta hydrochimica et hydrobiologica, 31(1), pp.9-18. doi: 10.1002/aheh.200390025