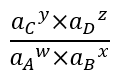Metals and Metalloids - Mobility in Groundwater
The mobility of metals and metalloids (collectively referred to as “metals” in this article) in groundwater is fundamental to assessment of risk these contaminants pose, as well as their remediation. Mobile contaminants are more likely than less mobile contaminants to reach environmental receptors at concentrations that exceed risk-based levels. Geochemical gradients (such as changes in pH, redox potential, and ionic strength over time and space in a plume) cause partitioning of the contaminants to aquifer solids via adsorption and/or precipitation. This partitioning controls the mobility of metal contaminants in groundwater.
Related Article(s):
- Metal and Metalloid Contaminants
- Monitored Natural Attenuation (MNA) of Metal and Metalloids
- Metal and Metalloids - Remediation
CONTRIBUTOR(S): Dr. Miles Denham
Key Resource(s):
- Scenarios Approach to Attenuation-Based Remedies[1]
- Aquatic Chemistry: An Introduction Emphasizing Chemical Equilibria in Natural Waters[2]
Introduction
Mobility of a contaminant is the rate at which the contaminant moves relative to the rate at which groundwater moves. Highly mobile contaminants move at rates near those of groundwater and travel from sources to receptors in the same timeframe as groundwater. Other contaminants are affected by geochemical reactions that cause them to partition to aquifer solids. The partitioning results in retardation of the contaminant transport relative to groundwater. The degree of retardation depends on the chemical nature of the contaminant, the chemical composition of the groundwater, and the mineralogy of the aquifer. Contaminants that are highly mobile under one set of aquifer conditions may be relatively immobile under different conditions[1].
Aqueous speciation reactions, adsorption, and precipitation control the partitioning of metals to aquifer solids, and hence their mobility. Adsorption and precipitation cause partitioning of the contaminant to aquifer solids and aqueous speciation reactions can strongly influence the extent to which adsorption and precipitation occur. These processes can be considered as chemical reactions in which constituents react to form products to the extent determined by equilibrium constants. Discussions of adsorption, precipitation and aqueous speciation can be found in most aqueous geochemistry textbooks (e.g.[2][3][4]) of and other references discuss how these apply to specific metals are available[1][5][6][7].
A discussion of reactions responsible for aqueous speciation, adsorption, and precipitation requires a brief discussion of equilibrium constants[8] and activity. Any reaction:
where w, x, y, and z are coefficients of the reaction stoichiometry and A, B, C, and D are chemical constituents, has an equilibrium constant that defines whether the reaction will tend to proceed to the right, to the left, or not at all. The important metric is this ratio:
where ai is the activity (effective concentration; thermodynamic activity) of constituent i. When this ratio is less than the equilibrium constant, the reaction will tend to proceed to the left and vice versa. If the ratio equals the equilibrium constant, the reaction is at equilibrium and will not proceed in either direction. Most college level introductory chemistry textbooks will have a complete discussion of equilibrium constants and activity[9].
Aqueous Speciation
Single, or free, dissolved ions of metals and metalloids can combine with other dissolved ions to form different dissolved species, called aqueous complexes, with chemical properties different from the original ions. Metal contaminants form aqueous complexes with ions that are relatively abundant in groundwater. For example, the dissolved mercury ion Hg+2 readily combines with the chloride ion under mildly acidic conditions by the reaction:
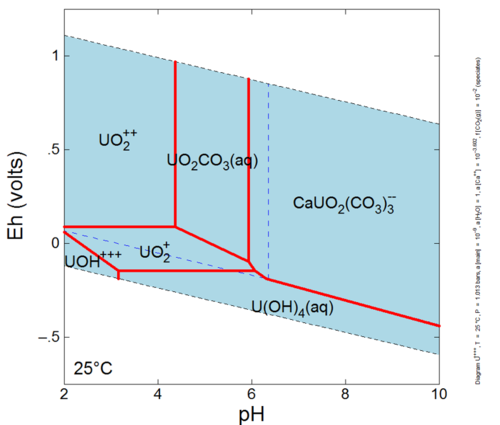
This reaction has an association, or equilibrium, constant that governs the proportions of Hg+2, Cl- and HgCl2o that can exist in solution at equilibrium. Mercury can form many other aqueous complexes and its distribution among these aqueous complexes is known as the aqueous speciation of mercury. Furthermore, aqueous speciation can change as conditions in the groundwater change. For example, let’s investigate the calculated aqueous speciation of uranium on an Eh-pH diagram (Fig. 1). The dominant species is different at different pH and Eh values (species activities are equal along the red lines between species dominance fields). Eh is a measure of the tendency of electrons to flow from one ion to another[3][4] while pH is an indicator of how acidic or basic the groundwater is. The aqueous speciation is also different when other components involved in speciation reactions have different activities (e.g., calcium and fugacity of CO2 in equilibrium with the system).
Speciation affects adsorption of a metal because different species are adsorbed to different degrees by aquifer minerals. This is because the charge and hydrated radii on aqueous species differs and these properties are important influences on adsorption. Aqueous speciation affects precipitation because the solubility of a mineral is defined by a single species. For example, if the solubility of the mineral anglesite is defined by the ion Pb+2, but Pb+2, is only a minor species in the aqueous speciation of lead, then the real solubility will be higher than that calculated from the “relation”:
Where Ksp is the solubility product constant, or the equilibrium constant for solid substance dissolving in an aqueous solution and “a” is the activity, or effective concentration, of each one of the different species in the reaction.
Oxidation-reduction chemistry plays an important role in aqueous speciation of certain metals such as arsenic (As), chromium (Cr), copper (Cu), mercury (Hg), selenium (Se), and uranium (U). These contaminants can exist in more than one oxidation state over the range of conditions found in contaminated groundwater. A change in oxidation state can profoundly change the adsorption behavior and solubility of a metal or metalloid (e.g., see the two chromium species in Figure 2 in MNA of Metal and Metalloid Contaminants). Reactions that change the oxidation state of metals are often kinetically limited, but can be catalyzed by microbial interactions[11][12]. Thus, for metals that are sensitive to oxidation-reduction potential, it is important to consider microbial activity and potential interactions with the contaminants when assessing mobility.
Adsorption
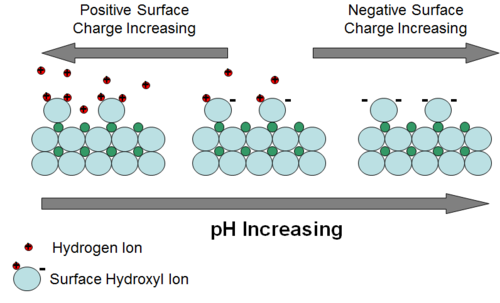
Adsorption can be defined as the partitioning of a constituent to the surface of an aquifer solid phase as a 2-dimensional “coating”[13]. For aqueous species carrying an electrical charge, partitioning is driven by electrostatic attraction and then potential bonding of the constituent to the surface of the solid. Uncharged (neutral) species may adsorb onto aquifer solid surfaces because they are repulsed, driven out of the aqueous phase, by energetically more favorable attraction of water molecules to each other. The surface of most aquifer minerals carries an electrical charge that varies with pH. For oxides and silicates, the charge is mostly the result of partially-bonded oxygen atoms at the surface. The resulting negative charge attracts positively charged ions. One of the most common and energetically favorable ions available to bond with the surface oxygens is the hydrogen ion. Hence, as pH of groundwater decreases and hydrogen ions are more abundant, more hydrogen ions approach surface oxygens, neutralizing the negative charge (Fig. 2). The pH at which all charge has been neutralized is called the zero point of charge (ZPC). At pH values below the ZPC, the mineral surface becomes positively charged. This variable surface charge behavior of aquifer minerals is an important control on adsorption of metal and metalloid contaminants. Those contaminants that exist in groundwater primarily as cations tend to adsorb more strongly as pH increases. Those that exist primarily as anions tend to adsorb more strongly as pH decreases.
Mechanistic theories of adsorption consider the structure of ions attracted to the surface of a mineral. In general, there are ions that are touching the mineral surface because they have lost some or all of their waters of solvation. There are also ions that retain their waters of solvation and exist in a diffuse layer that carries less of an electrical charge with distance from the mineral surface. Adsorption of ions that touch the mineral surface are bonded more strongly to the surface. This type of adsorption is referred to in various sources as inner-sphere adsorption or complexation, specific adsorption, or chemisorption (chemical adsorption). Adsorption of ions that retain their waters of solvation in the diffuse layer is referred to as outer sphere adsorption or complexation, non-specific adsorption, or physisorption (physical adsorption). Detailed discussion of adsorption can be found in Dzombak and Morel (1990)[15], Stumm (1992)[16], Stumm (1995)[17], and Sposito (1995)[18]. Reviews of adsorption can be found in Stumm and Morgan (1981)[2], Langmuir (1997)[3], and Drever (1997)[4].
Ion exchange can be treated as a type of adsorption but its definition varies in the literature. Certain minerals, such as smectite clays and zeolites, exchange cations whereas some minerals exchange anions between their crystal structure and groundwater. Ions also exchange at the layer of outer sphere complexes and in the diffuse layer of ions at the interface of groundwater. Broadly, ion exchange includes all cases where ions are exchanged between the surface of a mineral and the aqueous phase. A more restrictive definition of ion exchange is sometimes used in soil science that includes only “readily exchanged” ions as outer sphere complexes or in the diffuse layer[19][20][21]. To maintain electrical neutrality in the groundwater, the moles of charge exchanged must be equal. This can either mean: 1) ions of the same charge exchange in a stoichiometry that results in a zero net change of charge in the groundwater, or 2) cations and anions exchange in a stoichiometry that results in a zero net change of charge in the groundwater.
The capacity of aquifer solids to exchange cations or anions can be measured using standardized methods. These are good indicators of whether an aquifer can accommodate sufficient adsorption of contaminant metals. It must be remembered, however, that the measurements are made under a specific set of conditions. Adsorption may be less or more under the conditions of the contaminated aquifer. The effect of cation exchange capacity on the mobility of several metals is incorporated in the scenarios system for evaluating MNA for inorganics[1].
Precipitation
Precipitation of contaminant metals can be an effective attenuation mechanism. Precipitation differs from adsorption in that the contaminant is bound in a 3-dimensional structure of a mineral or an amorphous precursor to a mineral. The mineral can be composed of the contaminant bound to counterions from the groundwater. For example, lead can combine with the sulfate ion and form the mineral anglesite (PbSO4) by the reaction:
The equilibrium constant of this reaction, often referred to as the Ksp (solubility product)[21] is defined as:
where ai is the activity, or effective concentration, of the species i. When the product of the aPb+2 and aSO4-2 equals the Ksp, the groundwater is said to be saturated with anglesite. If the product exceeds the Ksp then it is thermodynamically favorable for anglesite to precipitate. Note that the Ksp refers specifically to the Pb+2 ion and the SO4-2 ion. Lead in other dissolved species such as PbCl2o, PbCO3o, or even PbSO4o is not considered. Hence, the aqueous speciation of a metal is important in controlling whether or not it will precipitate from groundwater. The measurement of the concentrations of lead and sulfate in groundwater provides the total dissolved concentration of each constituent that may be distributed among many species. Precipitation of minerals in aquifers is discussed in most aqueous geochemistry textbooks (e.g.[1][2][3]).
There are several special cases of precipitation. One, known as coprecipitation, is when the contaminant is a minor component of a precipitating mineral. A metal can be coprecipitated in a mineral either because it chemically resembles a primary component of the mineral or because it adsorbs to the surface of the mineral as precipitation occurs, becoming “trapped” as the mineral continues to precipitate. Another is surface precipitation, the nucleation and precipitation of one mineral on the surface of another. In this case adsorption of ions that compose the precipitating mineral causes activities of the pertinent ions to become high enough that the layer of water at the surface of the host mineral becomes saturated with the precipitating mineral. The precipitating mineral may be saturated at the surface of the host mineral, but undersaturated in the bulk groundwater. Surface precipitation is discussed in detail by Stumm (1992)[16], and briefly discussed in U.S. EPA. (2007)[12].
Microbial reactions can also affect the mobility of metals and metalloids by causing them to precipitate from groundwater[12]. Under strongly reducing conditions microbes catalyze the reduction of sulfate to sulfide. Metals such as zinc (Zn), lead (Pb), nickel (Ni), and cadmium (Cd), as well as redox sensitive arsenic (As) copper (Cu), and mercury (Hg), will readily precipitate as sulfide minerals[22]. Such reducing conditions are generally caused by a series of microbial reactions that deplete the system of oxidants such as oxygen, nitrate, and ferric iron. For other metals, like chromium (Cr) and uranium (U), the reducing conditions caused by microbes changes the oxidized and mobile forms (Cr(VI) and U(VI)) into reduced forms (Cr(III) and (UIV)) that precipitate as hydroxides or oxides.
Colloidal Transport
Contaminants can be transported in groundwater by colloidal particles and this can enhance the mobility of metals and metalloids[16][23][24][25]. This occurs when contaminants attach to mobile colloidal particles of minerals or when contaminants precipitate from groundwater, but remain mobile as colloidal particles. The mobility of colloidal particles depends on the surface charge of the particles relative to each other and relative to aquifer mineral surfaces. These are most sensitive to pH and ionic strength. Despite the widespread occurrence of contaminants associated with colloidal particles, it is unusual that the concentration of contaminant carried by colloidal particles is the primary cause for exceeding regulatory standards at an exposure point.
Simple Guide to Mobility of Metals in Groundwater
All concepts described above were incorporated into a “scenarios approach” guidance document[1] to help groundwater professionals to evaluate the mobility of several metals and inorganics (Fig. 2 in MNA of Metal and Metalloid Contaminants). It shows how three primary factors (oxidation/reduction potential (ORP); cation exchange capacity (CEC), and sediment iron oxide coatings and solids), combine with three secondary factors (pH, sulfur/sulfide, and total dissolved solids) to provide a semi-qualitative indicator of mobility.
References
- ^ 1.0 1.1 1.2 1.3 1.4 1.5 Truex, M., Brady, P., Newell, C.J., Rysz, M., Denham, M., Vangelas, K. 2011. The scenarios approach to attenuation-based remedies for inorganic and radionuclide contaminants. Savannah-River National Laboratory, U.S. Department of Energy. Report pdf
- ^ 2.0 2.1 2.2 2.3 Stumm, W., and Morgan J. J. 1981. Aquatic chemistry: An introduction emphasizing chemical equilibria in natural waters. John Wiley & Sons.
- ^ 3.0 3.1 3.2 3.3 Langmuir, D., 1997, Aqueous Environmental Geochemistry. Prentice-Hall, Inc. Upper Saddle River, NJ ISBN 978-0023674129.
- ^ 4.0 4.1 4.2 Drever, J.I., 1997. The Geochemistry of Natural Waters: Surface and Groundwater Environments. Prentice-Hall, Inc. ISBN 0132727900.
- ^ United States Environmental Protection Agency (USEPA), 2007. Monitored Natural Attenuation of Inorganic Contaminants in Groundwater, Volume 2 - Assessment for Non-Radionulcides Including Arsenic, Cadmium, Chromium, Copper, Lead, Nickel, Nitrate, Perchlorate, and Selenium, Edited by R.G. Ford, R.T. Wilkin, and R.W. Puls. U.S. Environmental Protection Agency, EPA/600/R-07/140. Report pdf
- ^ U.S. Environmental Protection Agency (USEPA), 2007. Monitored natural attenuation of inorganic contaminants in groundwater, Volume 3 Assessment for Radionuclides Including Tritium, Radon, Strontium, Technetium, Uranium, Iodine, Radium, Thorium, Cesium, and Plutonium-Americium, Edited by R.G. Ford and R.T. Wilkin. U.S. Environmental Protection Agency, EPA/600/R-10/093. Report pdf
- ^ Wilkin, R.T., 2007. Metal attenuation processes at mining sites. Ground Water Issue. Environmental Protection Agency, EPA/600/R-07/092. Report pdf
- ^ U.C. Davis, ChemWiki. The Equilibrium Constant. Contributor: Heather Voigt. Updated: July 12, 2016. Report pdf
- ^ Brown, T.L., H.E. LeMay, B.E. Bursten, and J.R. Burdge, 2015 13th Ed. Chemistry: The Central Science. Prentice Hall, Upper Saddle River, NJ. ISBN 978-0321910417.
- ^ Bethke, C.M. and S. Yeakel, 2015. The geochemist’s workbench®, Release 10.0. Latest version available at The Geochemist's Workbench
- ^ NABIR, 2003. Bioremediation of metals and radionuclides - what it is and how It works. LBNL-42595, Lawrence Berkeley National Laboratory for the Natural and Accelerated Bioremediation Research Program, Office of Science, U.S. Department of Energy. Report pdf
- ^ 12.0 12.1 12.2 United States Environmental Protection Agency (USEPA), 2007. Monitored natural attenuation of inorganic contaminants in groundwater, Volume 1 Technical basis for assessment, Edited by R.G. Ford, R.T. Wilkin, and R.W. Puls. U.S. Environmental Protection Agency, EPA/600/R-07/139. Report pdf
- ^ United States Environmental Protection Agency (USEPA), 1999. Understanding variation in partition coefficient, Kd, values, Volume 1 - The Kd model, methods of measurement, and application of chemical reaction codes. EPA 402-R-99-004A. Report pdf
- ^ ITRC, 2010. A Decision Framework for Applying Monitored Natural Attenuation Processes to Metals and Radionuclides, Interstate Technology and Regulatory Council, Technical/Regulatory Guidance AMPR-1. Report pdf
- ^ Dzombak, D.A. and Morel, F.M., 1990. Surface complexation modeling: hydrous ferric oxide. John Wiley & Sons. ISBN 0-471-63731-9.
- ^ 16.0 16.1 16.2 Stumm, W. 1992. Chemistry of the Solid-Water Interface - Processes at the Mineral-Water and Particle-Water Interface in Natural Systems. John Wiley & Sons, Inc., ISBN 0-471-57672-7.
- ^ Stumm, W. 1995. The inner sphere surface complex - A key to understanding surface reactivity. C.P. Huang, C.R. O’Melia, and J.J. Morgan (Eds.) Aquatic Chemistry - Interfacial and Interspecies Processes. American Chemical Society, Washington DC. doi: 10.1021/ba-1995-0244.ch001
- ^ Sposito, G. 1995. Adsorption as a problem in coordination chemistry - The concept of the surface complex. C.P. Huang, C.R. O’Melia, and J.J. Morgan (Eds.) Aquatic Chemistry - Interfacial and Interspecies Processes. American Chemical Society, Washington DC. doi: 10.1021/ba-1995-0244.ch002
- ^ Dzombak, D.A. and J.M. Hudson, 1995. Ion exchange - The contributions of diffuse layer sorption and surface complexation. C.P. Huang, C.R. O’Melia, and J.J. Morgan (Eds.) Aquatic Chemistry - Interfacial and Interspecies Processes. American Chemical Society, Washington DC. doi: 10.1021/ba-1995-0244.ch003
- ^ Bourg, I.C. and G. Sposito, 2011. Ion Exchange Phenomena. LBNL-4940E, Lawrence Berkeley National Laboratory.
- ^ 21.0 21.1 U.C. Davis, Chemwiki. Solubility Product Constant. Contributors: Kathryn Rashe, Lisa Peterson. Report pdf
- ^ Diels, L., Geets, J., Dejonghe, W., Van Roy, S., Vanbroekhoven, K., Szewczyk, A. and Malina, G., 2010, January. Heavy metal immobilization in groundwater by in situ bioprecipitation: comments and questions about efficiency and sustainability of the process. In Proceedings of the Annual International Conference on Soils, Sediments, Water and Energy (Vol. 11, Article 7).
- ^ McCarthy, J.F. and Zachara, J.M., 1989. Subsurface transport of contaminants. Environmental Science & Technology, 23(5), pp. 496-502. doi: 10.1021/es00063a001
- ^ Puls, R.W., R.M. Powell, D.A. Clark, and C.J. Paul, 1991. Facilitated transport of inorganic contaminants in groundwater: part II. colloidal transport. U.S Environmental Protection Agency EPA/600/M-91/040. Report pdf
- ^ Takala, M. and Manninen, P., 2006. Sampling and analysis of groundwater colloids. A literature review (No. POSIVA-WR--06-15). Posiva Oy.