Difference between revisions of "Munitions Constituents - IM Toxicology"
m (1 revision imported) |
m (1 revision imported) |
(No difference)
| |
Revision as of 20:05, 3 May 2018
The US military is phasing out traditional explosives in favor of insensitive munitions (IM) to reduce risks of accidental detonation. This article overviews recent results on the toxicology of some common IM components, namely 2,4-dinitroanisole (DNAN), nitrotriazelone (3-nitro-1,2,4-triazol-5-one, NTO), and nitroguanidine (NQ).
Related Article(s):
- Munitions Constituents
- Munitions Constituents - Deposition
- Munitions Constituents - Dissolution
- Munitions Constituents - Sorption
Key Resource(s):
- Recommendation of an occupational exposure level for PAX-21[1]
- Subchronic oral toxicity of 3-nitro-1,2,4-triazol-5-one (NTO) in rats[2]
Introduction
The US military is replacing many of traditional explosives with insensitive munitions (IM) to reduce risks of accidental detonation. Since these IM explosives (IMX) materials have not been in common use, considerable effort has been focused on understanding the toxicology of these materials. Some insensitive munition formulations use a combination of materials including 2,4-dinitroanisole (DNAN), nitrotriazelone (3-nitro-1,2,4-triazol-5-one, NTO), nitroguanidine (NQ), and Research Department Formula X (RDX).
2,4-Dinitroanisole (DNAN) Toxicity
Summary
The nitroaromatic DNAN has toxicity properties very similar to other compounds of that class. Briefly, DNAN appears to be less toxic than TNT and many other nitroaromatics in mammalian and aquatic organisms.
Mammalian
Oral toxicity of DNAN is similar to other nitroaromatics[1]. The median lethal dose (LD50, oral) in rats was 199 mg/kg in both sexes. Clinical signs of toxicity included decreased activity, breathing abnormalities, salivation, and soft stools[1]. One study reported an oral Approximate Lethal Dose (ALD) of 300 mg/kg in rats[3]. After 14 days of dosing, the primary non-lethal adverse events suggested that the nitro groups were causing anemia.
Genotoxicity
Genotoxicity testing of DNAN has had mixed results. DNAN tested positive in the Ames Salmonella histidine reversion test in strain TA100 without activation[4][5]. DNAN tested negative in Chinese Hamster Ovary (CHO) cells (AS52/XPRT) at concentrations up to 1.0 mg/ml with and without S9 activation[6]. DNAN genotoxicity was negative in the in vivo mouse micronucleus assay at exposures of 10-90 mg/kg in both males and females[1].
Ecotoxicity
Administration of DNAN to Japanese quail (Coturnix japonica) resulted in rapid development of cataracts. All quail receiving single oral doses of 120 or 150 mg/kg developed cataracts within 4 hours of treatment. Mortality was also noted in these groups with losses being 1 of 5 at the lower dose and 5 of 9 at the higher dose[7].
Acute and chronic aquatic toxicity bioassays conducted using standard fish (Pimephales promelas) and invertebrate (Ceriodaphnia dubia) indicated that acute toxicity was similar for the two species tested, with 48-hour lethal median concentrations (LC50) ranging from 37 to 42 mg/L DNAN. Chronic toxicity tests indicated that fish (7-day LC50 = 10 mg/L) were more sensitive to DNAN compared to invertebrate (no significant impact on survival at 24 mg/L).
When assessing the most sensitive chronic endpoints, the two test species had similar chronic toxicity, with lowest observable adverse impacts ranging from 10 to 12 mg/L DNAN and median effects on sublethal endpoints (growth, reproduction) ranging from 11 to 15 mg/L DNAN. Chronic no-effect concentrations ranged from approximately 6 to 8 mg/L DNAN, which is less than that reported for TNT[8][9]. In a 96-hour freshwater green algae (P. subcapitata) inhibition test, DNAN had an EC20 of 1.4 mg/L (concentration where 20% of maximum effect is observed). The results obtained for DNAN are similar to TNT (EC20 of 0.54 mg/L[10])
Stanley et al. (2015)[11] reported acute and chronic (28-day) toxicity of DNAN exposure to northern leopard frogs (Rana pipiens; (sic)). The 96-hour LC50 values from DNAN exposure were 24.3 mg/L (95% CI - 21.3-27.6 mg/L). The lowest observed effect concentration (LOEC) for mortality from 28-day exposures to DNAN was 2.4 mg/L. Changes in growth, swimming distance, and other non-lethal parameters did not differ from controls.
Nitrotriazelone (NTO) Toxicity
Summary
Generally, NTO is much less toxic from oral exposures than other explosive munitions (EMs). High oral concentrations in mammalian models have shown the most sensitive outcome to be low sperm production through direct toxic mode of action to germ cells in males. Aquatic toxicity is largely due to the acidic nature of NTO when added to water.
Mammalian
NTO has very low acute oral toxicity. The oral median lethal dose (LD50) for NTO is >5000 mg/kg in both the rat and mouse systems[12].
Results from a 14-day subacute (between acute and chronic) oral toxicity study of NTO in rats were significantly decreased testes weights in the high-dose groups (≥500 mg/kg-day[2][13], but not the lower dose groups.
The most sensitive effect from a 90-day oral gavage study (feeding by means of a tube passed into the stomach) in rats of 0, 30, 100, 315, and 1000 mg NTO/kg-day found that testes and epididymides weights were reduced in the 315 and 1000 mg/kg-day exposures. NTO had no effect on mortality, food consumption, body weight, or neurobehavioral parameters. Moderate to severe testicular hypoplasia (underdevelopment), characterized by interstitial degeneration and loss of spermatogenic epithelium in the seminiferous tubules, was observed in the testes in 86% and 100% of males from the 315 and 1000 mg/kg-day dose groups, respectively. Epididymal aspermia was also observed at these dose levels[2][13]. The testicular effects were the most sensitive adverse effect and were used to derive a BMDL10 (benchmark lower confidence limit yielding a 10% increase in risk) of 40 mg/kg-d. Exposures in mice show similar results[14]. Reproductive studies have not found a relationship between NTO exposures and changes in offspring production; however, histological changes in testes and epididimydes remain consistent[15]. Results of investigating changes in endocrine disruption have largely been negative[16].
Genotoxicity
Test results examining damage to genetic information within a cell (genotoxicity) were negative in Salmonella at levels up to 500 µg/plate without activation and up to 5000 µg/plate with activation. In E. coli, results were also negative at maximum concentrations up to 2500 µg/plate without activation and 5000 µg/plate with activation[17].
NTO was also evaluated in the L5178Y TK+/˗ mouse lymphoma mutagenesis assay. Cells were treated with NTO at concentrations up to 5000 µg/mL, both with and without activation. Results of the assay were negative, either with or without activation[17].
NTO was tested in Chinese hamster ovary (CHO) cells for clastogenicity. The test was conducted both with and without exogenous metabolic activation at concentrations up to 5000 µg/mL; results were negative[17]. NTO was negative in the SOS chromotest[10].
Ecotoxicity
The 48-hour survival of Pimephales promelas was examined in containing NTO at concentrations ranging from 0 to 5.0 %. The LC50 was 1.14 g/L calculated using the Trimmed-Spearman Karber method[18][19].
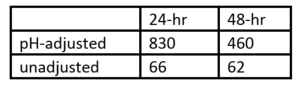
Ceriodaphnia dubia was used in a 7-day survival and reproduction study and the unicellular green algae Selenastrum capricornutum in a 96-hour growth inhibition study. In the definitive 7-day exposure study, the IC50-value was found to be 57 mg/L. The NOEC and LOEC values were found to be 34 mg/L and 66 mg/L, respectively. No eggs were produced at 262 mg/L, and at 133 mg/L eggs were produced but failed to develop[20], despite the lack of mortality at all concentrations up to 523 mg/L. System pH impacts the results (Table 1).
Nitroguanidine (NQ) Toxicity
Summary
NQ generally is the least toxic of all three IM compounds. No acute toxicity or mutagenic effects have been observed. However, more research is needed to understand the potential for aquatic toxicity of environmental breakdown products that are likely short-lived.
Mammalian
Nearly all studies conducted to-date suggest very low toxicity from exposures to NQ. The LD50 is 3850 mg/kg in mice and 3120 mg/kg in guinea pig. Mortality is the result of respiratory cyanosis. The LD50 in rats is >5000 mg/kg[21][22][23].
Subacute and subchronic (repeats over short period) oral toxicity of NQ in male and female rats exposed through the diet (0, 100, 316, or 1000 mg/kg-day for 90 days) indicated that food consumption was reduced and water consumption increased, with no other toxicity indicators[24][25]. Blood samples exhibited no abnormalities that could be attributed to NQ exposure. Microscopic examination of tissues from the control and 1000 mg/kg-day dose group animals suggested no lesions attributable to NQ exposure.
The 90-day subchronic oral toxicity of NQ using ICR (Institute of Cancer Research) mice exposed in to diet dose levels of 0, 100, 316 or 1000 mg/kg-day for 90 days indicated no effect on food consumption or weight gain; there was a dose-dependent increase in water consumption. Several serum chemistry parameters did exhibit differences compared to control values, but these changes were isolated occurrences with no consistent dose-related trends reported. Microscopic examination of tissues from the control and 1000 mg/kg-day dose group suggested no lesions attributable to the administration of NQ. The findings of increased water consumption suggest that NQ, which is excreted unchanged in mouse urine, may be acting as an osmotic diuretic. Higher brain-to-body weight ratios in male mice at 1000 mg/kg-day NQ supported a 316 mg/kg-day no adverse effect level (NOAEL)[26].
Genotoxicity
NQ was not mutagenic in the Ames assay using Salmonella typhimurium strains, nor was it mutagenic for mouse lymphoma cells in the presence or absence of rat hepatic homogenates[27][28][29]. NQ-associated recombinant activity was not observed in Saccharomyces cerevisiae[28], and it was negative in dominant lethal assays with rats and mice[30]. NQ did not induce sister chromatid exchange in CHO cells at concentrations up to 3.9 mg/ml. DNA repair tests using E. coli (10 mg/plate) indicated no activity of NQ[31][28].
Ecotoxicity
Fish exposed to NQ for 96 hours included fathead minnows (Pimephales promelas), bluegill (Lepomis macrochirus), Channel catfish (Ictalurus punctatus), and rainbow trout (Salmo gairdneri). Invertebrates were exposed for 48-hours and included Daphnia magna, amphipods (Hyallela azteca and Gammarus minus), midge larvae (Paratanytarsus dissimilis), and aquatic worms (Lumbriculus variegatus). The acute toxicity of NQ was very low; fewer than 50% of the exposed organisms exposed died at concentrations up to the solubility limit of NQ in water (1700 mg/mL at 12oC for trout to about 3000 mg/L at 22oC for most other species). The alga (Selenastrum capricornutum) was slightly more sensitive, with 120-hour EC50’s of about 2000 mg/L. Complete photolysis of NQ with ultraviolet light greatly increased toxicity, with LC50/EC50 values decreasing to 20-35 mg/L (nominal concentration estimates).
Burrows et al. (1988)[32] investigated the photolytic toxicity further and reported NQ is readily degraded in water by ultraviolet and natural sunlight. The principal end products of photolysis from unbuffered NQ solutions are guanidine, urea, and nitrite ion, with lesser quantities of cyanoguanidine, nitrate ion and ammonia, accounting for 80% of the carbon and virtually all of the nitrogen. Nitrosoguanidine is an early intermediate, which is even more readily photolyzed, to guanidine. Photolysis of NQ at pH 10 proceeds at nearly the same rate as the unbuffered reaction, but the product mix is different; less than 25% of NQ carbon is accounted for as urea, guanidine and cyanoguanidine. N2 is a significant product.
All the identified photolysis products of NQ except urea are more toxic to aquatic organisms than the parent compound. However, only nitrite ion is present at a level high enough to account for the greatly enhanced toxicity of photolyzed NQ. It is highly unlikely that wastewaters discharged to a body of moving water could present a hazard to aquatic life unless the NQ levels substantially exceeded the present National Pollutant Discharge Elimination System (NPDES) daily average limit of 25 mg/L for Sunflower Army Ammunition Plan, given the photolytic half-life of NQ and the dilution that would take place.
In a 96-hour freshwater green algae (P. subcapitata) inhibition test, NQ had an EC20 of 760 mg/L[10].
References
- ^ 1.0 1.1 1.2 1.3 Dodd, D.E. and McDougal, J.N., 2002. Recommendation of an occupational exposure level for PAX-21. AFRL-HE-WP-TR-2001-0103. Man-Tech Geo-Centers Joint Venture, Operational Toxicology Branch (AFRL/HEST). U.S. Air Force Armstrong Laboratory. Wright-Patterson Air Force Base, OH.
- ^ 2.0 2.1 2.2 USAPHC, L.C.B. Crouse, J. T. Houpt, A. O'Neill, M.R. Way, T.L. Hanna, and M.J. Quinn, 2010. Toxicology Study No. 85-XC-0A6W-08, Protocol No. 0A6W-38-08-02-01, Subchronic oral toxicity of 3-nitro-1,2,4-triazol-5-one (NTO) in rats. U.S. Army Public Health Command, Toxicology Portfolio, Aberdeen Proving Ground, MD 21010-5403.
- ^ USAPHC, Lent, E.M., Crouse, L.C., Hanna, T. and Wallace, S.M., 2012. The subchronic oral toxicity of 2, 4-dinitroanisole (DNAN) in rats (No. USAPHC-87-XE-0DBP-10). Army Public Health Command Aberdeen Proving Ground MD. Report pdf
- ^ McMahon, R.E., Cline, J.C. and Thompson, C.Z., 1979. Assay of 855 test chemicals in ten tester strains using a new modification of the Ames test for bacterial mutagens. Cancer Research, 39(3), pp. 682-693. Report pdf
- ^ Chiu, C.W., Lee, L.H., Wang, C.Y. and Bryan, G.T., 1978. Mutagenicity of some commercially available nitro compounds for Salmonella typhimurium. Mutation Research/Genetic Toxicology, 58(1), pp. 11-22. doi:10.1016/0165-1218(78)90090-3
- ^ Dodd, D.E., S. Sharma, and G.M. Hoffman, 2002. Genotoxicity and 90-day developmental toxicity studies on an explosive formulation. Toxicologist 66, 267
- ^ Takahashi, K.W., Saito, T.R., Amao, H., Kosaka, T., Obata, M., Umeda, M. and Shirasu, Y., 1988. Acute reversible cataract due to nitrocompounds in Japanese quail (Coturnix coturnix japonica). Jikken dobutsu. Experimental animals, 37(3), pp. 239-243.
- ^ Kennedy, A.J., Lounds, C.D., Melby, N.L., Laird, J.G., Winstead, B., Brasfield, S.M. and Johnson, M.S., 2013. Development of environmental health criteria for insensitive munitions: Aquatic ecotoxicological exposures using 2, 4-dinitroanisole (No. ERDC/EL-TR-13-2). Engineer Research and Development Center, Vicksburg, MS Environmental Lab. Report pdf
- ^ Kennedy, A.J., Laird, J.G., Lounds, C., Gong, P., Barker, N.D., Brasfield, S.M., Russell, A.L. and Johnson, M.S., 2015. Inter‐and intraspecies chemical sensitivity: A case study using 2, 4‐dinitroanisole. Environmental Toxicology and Chemistry, 34(2), pp. 402-411. doi: 10.1002/etc.2819
- ^ 10.0 10.1 10.2 DRDC, J. Hawari., 2011. Annual report 2010-2011. Environmental fate and ecological impact of emerging energetic chemicals (DNAN and its Amino-Derivatives, NTO, NQ, FOX-7, and FOX-12). NRC# 53363, Defense Research and Development Canada, National Research Council of Canada, Montréal, Québec.
- ^ Stanley, J.K., Lotufo, G.R., Biedenbach, J.M., Chappell, P. and Gust, K.A., 2015. Toxicity of the conventional energetics TNT and RDX relative to new insensitive munitions constituents DNAN and NTO in Rana pipiens tadpoles. Environmental Toxicology and Chemistry, 34(4), pp. 873-879. doi: 10.1002/etc.2890
- ^ London, J.E. and Smith, D.M., 1985. Toxicological study of NTO (No. LA-10533-MS). Los Alamos National Lab., NM (USA)
- ^ 13.0 13.1 Crouse, L.C., Lent, E.M. and Leach, G.J., 2015. Oral Toxicity of 3-Nitro-1, 2, 4-triazol-5-one in Rats. International Journal of Toxicology, 34(1), 55-66. doi: 10.1177/1091581814567177
- ^ Mullins, A.B., Despain, K.E., Wallace, S.M., Honnold, C.L. and May Lent, E., 2016. Testicular effects of 3-nitro-1, 2, 4-triazol-5-one (NTO) in mice when exposed orally. Toxicology Mechanisms and Methods, 26(2), pp. 97-103. doi:10.3109/15376516.2015.1118175
- ^ USAPHC, L.C.B. Crouse, E.M. Lent, T.L. Hanna, and S.M. Wallace, 2013. Repeated-Dose and Reproductive/Developmental Toxicity of NTO in the Rat. U.S. Army Public Health Command, Toxicology Portfolio, Aberdeen Proving Ground, MD.
- ^ USAPHC, V. H. Adams, 2012. In Vitro Endocrine Disruption Screening of 3-nitro-1,2,4-triazol-5-one (NTO). Toxicology Report No. S.0002745-12. U.S. Army Public Health Command, Toxicology Portfolio, Aberdeen Proving Ground, MD.
- ^ 17.0 17.1 17.2 Reddy, G., Song, J., Kirby, P., Lent, E.M., Crouse, L.C. and Johnson, M.S., 2011. Genotoxicity assessment of an energetic propellant compound, 3-nitro-1, 2, 4-triazol-5-one (NTO). Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 719(1), 35-40. doi:10.1016/j.mrgentox.2010.11.004
- ^ BAE Systems, 2007. Biomonitoring Retest of NTO Aquatic Toxicity in Pimephales promelas. West Stone Drive, Kingsport, TN 37660-9982.
- ^ BAE Systems Ordnance Systems, Inc. 2007. Material Safety Data Sheet (MSDS)-NTO. In 4509 West Stone Drive, Kingsport, TN 37660-9982.
- ^ Haley, M.V., Kuperman, R.G. and Checkai, R.T., 2009. Aquatic toxicity of 3-nitro-1, 2, 4-triazol-5-one (No. ECBC-TR-726). Edgewood Chemical Biological Center (ECBC), Aberdeen Proving Ground, MD. Report pdf
- ^ Brown, L.D., Wheeler, C.R. and Korte Jr, D.W., 1988. Acute Oral Toxicity of Nitroguanidine in Male and Female Rats (No. LAIR-264). Letterman Army Inst. of Research Presidio of San Francisco, CA. Report pdf
- ^ Hiatt, G.F.S., Sano, S. K., Wheeler, C. R., and Korte, D.W., Jr., 1988. Acute oral toxicity of nitroguanidine in mice (No. LAIR-265). Letterman Army Institute of Research, Presidio of San Francisco, CA. Report pdf
- ^ Lewis, R.J., 2004. Nitroguanidine in SAX’s dangerous properties of industrial materials. New York, John Wiley & Sons, Inc., Scientific, Technical and Medical Division. doi: 10.1002/0471701343
- ^ Morgan, E.W., Brown, L.D., Lewis, C.M., Dahlgren, R.R. and Korte Jr, D.W., 1988. Fourteen-day subchronic oral toxicity study of nitroguanidine in rats (No. LAIR-272). Letterman Army Institute of Research, Presidio of San Francisco, CA. Report pdf
- ^ Morgan, E.W., Zaucha, G.M., Lewis, C.M., Makovec, G.T. and Pearce, M.J., 1988. Ninety-day subchronic oral toxicity study of nitroguanidine in rats (No. LAIR-306). Letterman Army Institute of Research Presidio of San Francisco, CA. Report pdf
- ^ Frost, D.F., Morgan, E.W., Letellier, Y., Pearce, M.J. and Ferraris, S., 1988. Ninety-day subchronic oral toxicity study of nitroguanidine in mice (No. LAIR-319). Letterman Army Institute of Research, Presidio of San Francisco, CA. Report pdf
- ^ Ishidate, M. and Odashima, S., 1977. Chromosome tests with 134 compounds on Chinese hamster cells in vitro-a screening for chemical carcinogens. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 48(3-4), 337-353. doi: 10.1016/0027-5107(77)90177-4
- ^ 28.0 28.1 28.2 McGregor, D.B., Riach, C.G., Hastwell, R.M. and Dacre, J.C., 1980. Genotoxic activity in microorganisms of tetryl, 1, 3‐dinitrobenzene and 1, 3, 5‐trinitrobenzene. Environmental Mutagenesis, 2(4), 531-541. doi: 10.1002/em.2860020411
- ^ Sebastian, S.E. and D.W. Korte, 1988. Mutagenic potential of Gguanidine Nitratenitrate. Technical Report No. 260. Toxicology Series 107 (ADA155040). Letterman Army Institute of Research, Presidio of San Francisco, CA.
- ^ Brusick, D. and Matheson, D.W., 1978. Mutagen and Oncogen Study on Nitroguanidine. Litton Bionetics Inc Kensington MD. Report pdf
- ^ Harbell, J.W. and Korte Jr, D.W., 1987. Mutagenic potential of nitroguanidine in the mouse lymphoma forward mutation assay (No. LAIR-252). Letterman Army Institute of Research, Presidio of San Francisco, CA. Report pdf
- ^ Burrows, W.D., Schmidt, M.O., Chyrek, R.H. and Noss, C.I., 1988. Photochemistry of aqueous nitroguanidine (No. USABRDL-TR-8808). Army Biomedical Research and Development Lab, Fort Detrick, MD. Report pdf