PFAS Transport and Fate
The transport and fate of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) in the environment is controlled by the nature of the PFAS source, characteristics of the individual PFAS, and environmental conditions where the PFAS are present. Transport, partitioning, and transformation are the primary processes controlling PFAS fate in the environment. PFAS compounds can also be taken up by both plants and animals, and in some cases, bioaccumulate through the food chain. Understanding PFAS transport and fate is necessary for evaluating the potential risk from a PFAS release and for predictions about PFAS occurrence, migration, and persistence, and about the potential vectors for exposure. This knowledge is important for site characterization, identification of potential sources of PFAS to the site, development of an appropriate conceptual site model (CSM), and selection and predicted performance of remediation strategies.
Related Article(s):
Contributor(s):
Dr. Hunter Anderson and Dr. Mark L. Brusseau
Key Resource(s):
- Assessing the Potential Contributions of Additional Retention Processes to PFAS Retardation in the Subsurface. Brusseau 2018 (manuscript).[2]
Introduction
The transport and fate of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) is a rapidly evolving field of science, with many questions that are not yet resolved. Much of the currently available information is based on a few well-studied PFAS compounds. However, there is a large number and variety of PFAS with a wide range of physical and chemical characteristics that affect their behavior in the environment. The transport and fate of some PFAS could differ significantly from the compounds studied to date. Nevertheless, information about the behavior of some PFAS in the environment can be ascertained from the results of currently available research.
PFAS transport and fate in the environment is controlled by the nature of the PFAS source, characteristics of the individual PFAS, and environmental conditions where the PFAS are present. Perfluoroalkyl acids (PFAAs) (see PFAS for nomenclature) are strong acids and are anionic in the environmentally-relevant pH range. They are extremely persistent in the environment and do not degrade or transform under typical environmental conditions. Polyfluoroalkyl substances (see PFAS for nomenclature) include compounds that have the potential to degrade to PFAAs. These compounds are commonly referred to as PFAA precursors or just ‘precursors’. Because some polyfluoroalkyl substances can degrade into PFAA via biotic or abiotic degradation pathways, PFAAs are sometimes referred to as “terminal PFAS” or “terminal degradation products”. The most important molecular properties controlling PFAA transport are the carbon chain length and functional moieties of the headgroups (e.g., sulfonate, carboxylate). The molecular properties of PFAA precursors are more varied, with different carbon chain lengths, headgroups and ionic states[3][4] (see PFAS). All of these properties can influence transport and fate of PFAA precursors in the environment.
Important environmental characteristics include the nature of the source (mode of input into the environment), the length of time that the source was active, and the magnitude of the input, as well as precipitation and infiltration rates, depth to groundwater, surface water and groundwater flow rates and interactions, prevailing atmospheric conditions, the properties of the porous-media (e.g., soil and sediment) and aqueous solution, microbiological factors, and the presence of additional fluid phases such as air and non-aqueous phase liquids (NAPLs) in the vadose zone and water-saturated source. In the subsurface, soil characteristics (texture, organic carbon content, clay mineralogy, metal-oxide content, solid surface area, surface charge, and exchange capacity) and solution characteristics (pH, redox potential, major ion chemistry, and co-contaminants) can influence PFAS transport and fate.
PFAS Transport and Fate Processes
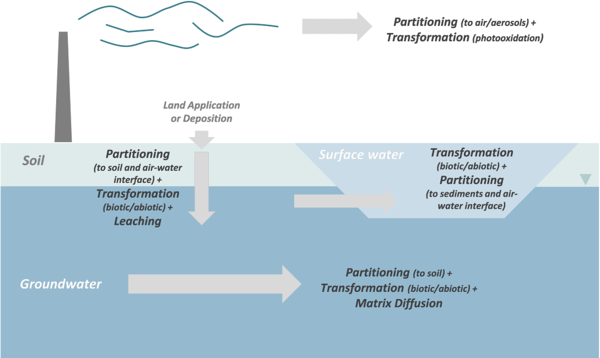
Transport, partitioning, and transformation are the primary processes controlling PFAS fate in the environment (Figure 1). PFAS compounds can also be taken up by both plants and animals, and in some cases, bioaccumulate through the food chain. However, PFAS uptake and bioaccumulation is not discussed in this article (see “Environmental Concern” section of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS)).
- Transport: PFAS can be transported substantial distances in the atmosphere[5], surface water[6], soil[7], and groundwater[8]. The primary mechanisms controlling PFAS transport are advection and dispersion, similar to other dissolved compounds. For additional information on transport in groundwater, see Advection and Groundwater Flow and Dispersion and Diffusion.
- Partitioning: Partitioning of PFAS between the mobile and immobile phases is one of the most important processes controlling the rate of migration in the environment. The primary mobile phases are typically air and water. Relatively immobile phases include stream sediments, soils, aquifer material, NAPLs, and interfaces between different phases (air-water, NAPL-water). Partitioning of a significant portion of the PFAS mass into an immobile phase increases the amount of material stored in the system and slows the apparent rate of migration in the mobile phase – a phenomenon that has been observed in field metadata[9].
- Transformation: Transformation of PFAS is controlled by the molecular structure of the individual compounds. Perfluorinated compounds, including PFAAs, are resistant to abiotic and biotic transformation reactions under typical conditions and highly persistent in the environment. In contrast, precursors can be transformed by both abiotic and biotic processes, often resulting in the production of so-called “terminal” PFAA daughter products.
Transport and Partitioning in the Atmosphere
Air serves as a transport media for PFAS, particularly for uncharged polyfluorinated PFAS. Airborne PFAS transport contributes to global distribution and can lead to localized deposition to soils and surface water in the vicinity of emission sources[10][11][12][13].
PFAAs, which are ionic and possess a negative charge under ambient environmental conditions, are far less volatile than many other groundwater contaminants. An online database of vapor pressures and Henry’s Law constants for different PFAS, including PFAAs, is maintained by the Interstate Technology Regulatory Council[1]. In general, vapor pressures of PFAS are low and water solubilities are high, limiting partitioning from water to air[1]. However, under certain conditions, particularly within industrial stack emissions, PFAS can be transported through the atmosphere in both the gas phase and associated with fugitive particulates. In particular, volatile compounds including fluorotelomer alcohols (FTOHs) may be present in the gas phase, whereas, PFAAs can aerosolize and be transported as particulates[5]. In addition, precursors can be transformed to PFAAs in the atmosphere, which can result in PFAA deposition. Short-range atmospheric transport and deposition can result in PFAS contamination in terrestrial and aquatic systems near points of significant emissions, impacting soil, groundwater, and other media of concern[14][15]. Releases of ionic PFAS from factories are likely tied to particulate matter, which settle to the ground in dry weather and are also wet-scavenged by precipitation[16]. The impact of other potential sources, such as combustion emissions or wind-blown fire-fighting foam from fire training and fire response sites, on the fate and transport of PFAS in air may need to be assessed.
Long-range transport processes are responsible for the wide distribution of neutral and ionic PFAS across the Earth as evidenced by their occurrence in biota, surface snow, ice cores, seawater, and other environmental media in regions as remote as the Arctic and Antarctic[17][18]. Distribution of PFAS to remote regions far removed from direct industrial input is believed to occur from both: a) long-range atmospheric transport and subsequent degradation of volatile precursors; and b) transport via ocean currents and release into the air as marine aerosols (sea spray)[19][20].
Transport and Partitioning in Aqueous Systems
PFAS adsorb from water to a variety of solid materials including organic materials, clay minerals, metal oxides, and granular activated carbon[21]. This process is thought to occur through two primary mechanisms: 1) sorption to organic-carbon components of the solids; and 2) electrostatic (and other) interactions with inorganic constituents of the solids, including clay minerals and metal-oxides[22][23]. The relative contribution of each mechanism varies depending on surface chemistry and other geochemical factors, as well as the molecular properties of the PFAS. In general, the impact of electrostatic interactions with charged soil constituents is more important for PFAS than non-polar, hydrophobic organic contaminants (e.g. hydrocarbons, chlorinated solvents). Adsorption of PFAS by solids is often nonlinear, with greater sorption at lower solute concentrations. The impacts of adsorption kinetics and their potential reversibility on PFAS transport have not yet been examined for most PFAS compounds.
Sorption of hydrocarbons, chlorinated solvents and other hydrophobic organics is often controlled the by organic-carbon components of the solid phase (see Sorption of Organic Contaminants). However, studies of PFAS sorption to solid phase organic carbon have reported conflicting results. In a study of field sites with aqueous film-forming foam (AFFF, a type of fire-fighting foam) releases, solid phase organic carbon content was found to significantly influence PFAS soil-to-groundwater concentration ratios. Statistical modeling was then used to derive apparent organic carbon partition coefficients for 18 different PFAS[9]. A recent compilation of published organic carbon partition coefficients found a good correspondence to PFAS molecular structure[24]. However, other studies have shown a general lack of correlation between solid phase partition coefficients and organic carbon[25]. It is possible that greater variability may be observed for broader data sets that incorporate different ranges of PFAS concentrations, different solution conditions, different measurement methods, and field-based data which often have less well-defined conditions and may also be influenced by other retention processes[9][24].
Most solids present in the environment contain both fixed-charged (negative) and variably charged surfaces. At neutral to high pH, variably charged clay minerals have a net-negative charge. As a result, negatively charged PFAAs do not strongly interact electrostatically in most soils, although as the soil pH decreases electrostatic sorption would be expected to increase in soils with variably charged clay minerals. Cationic and zwitterionic precursors are expected to be more strongly sorbed than anionic PFAAs in most environments due to well-established cation exchange reactions. Other factors, including ionic strength, composition, and the presence of co-solutes, can affect adsorption of PFAS[26][27][28][22][23].
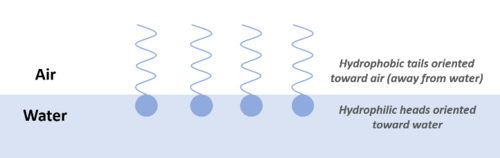
Most PFAS compounds act as surface-active agents (or surfactants) due to the presence of a hydrophilic headgroup and a hydrophobic tail. The hydrophilic headgroup will preferentially partition to the aqueous phase and the hydrophobic tail will preferentially partition to the non-aqueous phase (air or organic material). As a result, PFAS tend to accumulate at interfaces (air-water, water-NAPL, water-solid) (Figure 2). This tendency to accumulate at interfaces can influence transport in the atmosphere (on water droplets and hydrated aerosols), in the vadose or unsaturated zone at air-water interfaces, in the presence of NAPLs, and in wastewater treatment systems[2][29].
In theoretical and experimental studies of transport in unsaturated porous media, adsorption at the air-water interface increased PFOS and PFOA retention[2][30][31], contributing approximately 20% to 80% of total retention in sands and soil. The impact of oil-water interfacial adsorption on PFAS transport was also quantitatively characterized in recent studies and shown to contribute to total retention on a similar scale as air-water interfacial adsorption[2][31]. These processes may result in increased PFAS mass retained in NAPL source zones, increased PFAS sorption with the resulting retardation of transport, and greater persistence of dissolved PFAS in the environment.
Transformation
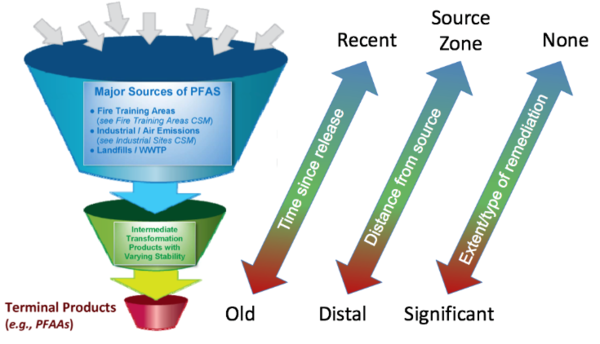
Certain polyfluorinated substances have the potential to transform to other PFAS, with PFAAs as the typical terminal daughter products. These polyfluorinated substances are often referred to as “precursors”. The transformation potential of polyfluorinated precursors is influenced by the presence, location, and number of carbon-hydrogen (C-H) bonds and potentially carbon-oxygen (C-O) bonds throughout the carbon chain. Specifically, PFAS with C-H bonds are subject to a variety of biotic and abiotic reactions that ultimately result in the formation of PFAAs with perfluorinated carbon chains of the same length or shorter than the initial polyfluorinated precursor[32][33][34][8].
Transformation studies published to date have tested only a small subsample of possible precursors and, therefore, much uncertainty exists regarding 1) the extent to which precursor transformation occurs on a global scale, 2) which environmental compartments represent the majority of transformation, 3) relevant environmental conditions that affect transformation processes, and 4) transformation rates and pathways. Nevertheless, a portion of the precursors are expected to transform to PFAAs over time as shown in Figure 3.
Precursors can be transformed by a variety of abiotic processes including hydrolysis, photolysis, and oxidation. Hydrolysis of some precursors, followed by subsequent biotransformation, can produce perfluoroalkyl sulfonates (PFSAs). An important example is the production of PFOS from perfluorooctane sulfonyl fluoride (POSF)[35]. Other hydrolysis reactions produce perfluoroalkyl carboxylates (PFCAs). At neutral pH, the hydrolysis of fluorotelomer-derived polymeric precursors results in the formation of monomeric precursors of PFOA and other PFAAs with half-lives of 50 to 90 years[36]. Oxidation of precursors by hydroxyl radicals can occur in natural waters, with the fluorotelomer-derived precursors being oxidized relatively rapidly[37][38]. Evidence of aerobic biotransformation is provided from studies of PFAS composition throughout the continuum of wastewater treatments[39], from field studies at AFFF-impacted sites[32][33][34][8], and from microcosm experiments. In general, the literature on aerobic biotransformation collectively demonstrates or indirectly supports the following conclusions as summarized in ITRC 2020[1]:
- Numerous aerobic biotransformation pathways exist with relatively rapid kinetics
- All polyfluorinated precursors studied to date have the potential to aerobically biotransform to PFAAs
- Aerobic biotransformation of various fluorotelomer-derived precursors exclusively results in the formation of PFCAs, including PFOA, without necessarily the conservation of chain-length
- Aerobic biotransformation of various electrochemical fluorination-derived precursors primarily results in the formation of PFAAs, including PFOS, with the conservation of chain-length
Precursor transformation can complicate CSMs (and risk assessments) and should be considered during comprehensive site investigations. For example, atmospheric emissions of volatile precursors can result in long-range transport where subsequent transformation and deposition can result in detectable levels of PFAAs in environmental media independent of obvious point-sources[40]. With respect to site-related precursors, transformation of otherwise unmeasured PFAS into detectable PFAAs is obviously relevant to site investigations to the extent transformation occurs after initial site characterization efforts or if past remedial efforts have accelerated in situ transformation rates[33]. Additionally, differential transport rates between precursor PFAS and the corresponding terminal PFAA could also confound CSMs if transformation rates are slower than transport rates as has been suggested[8]. To account for otherwise unmeasurable precursors, several surrogate analytical methods have been developed. See PFAS Sampling and Analytical Methods for additional detail.
References
- ^ 1.0 1.1 1.2 1.3 Interstate Technology and Regulatory Council (ITRC), 2020. Technical/Regulatory Guidance: Per- and Polyfluoroalkyl Substances (PFAS), PFAS-1. ITRC, PFAS Team, Washington DC. Free Download from ITRC. Report.pdf
- ^ 2.0 2.1 2.2 2.3 Brusseau, M.L., 2018. Assessing the Potential Contributions of Additional Retention Processes to PFAS Retardation in the Subsurface. Science of the Total Environment, 613-614, pp. 176-185. DOI: 10.1016/j.scitotenv.2017.09.065 Author’s Manuscript
- ^ Buck, R.C., Franklin, J., Berger, U., Conder, J.M., Cousins, I.T., de Voogt, P., Jensen, A.A., Kannan, K., Mabury, S.A., and van Leeuwen, S.P.J., 2011. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integrated Environmental Assessment and Management, 7(4): pp. 513-541. DOI: 10.1002/ieam.258 Open Access Article
- ^ Wang, Z., DeWitt, J.C., Higgins, C.P., and Cousins, I.T., 2017. A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environmental Science and Technology, 51(5), pp. 2508-2518. American Chemical Society. DOI: 10.1021/acs.est.6b04806 Free Download from ACS
- ^ 5.0 5.1 Ahrens, L., Harner, T., Shoeib, M., Lane, D.A. and Murphy, J.G., 2012. Improved Characterization of Gas–Particle Partitioning for Per- and Polyfluoroalkyl Substances in the Atmosphere Using Annular Diffusion Denuder Samplers. Environmental Science and Technology, 46(13), pp. 7199-7206. DOI: 10.1021/es300898s Free download available from ResearchGate.
- ^ Taniyasu, S., Yamashita, N., Moon, H.B., Kwok, K.Y., Lam, P.K., Horii, Y., Petrick, G. and Kannan, K., 2013. Does wet precipitation represent local and regional atmospheric transportation by perfluorinated alkyl substances? Environment International, 55, pp. 25-32. DOI: 10.1016/j.envint.2013.02.005
- ^ Bräunig, J., Baduel, C., Heffernan, A., Rotander, A., Donaldson, E. and Mueller, J.F., 2017. Fate and redistribution of perfluoroalkyl acids through AFFF-impacted groundwater. Science of the Total Environment, 596, pp. 360-368. DOI: 10.1016/j.scitotenv.2017.04.095
- ^ 8.0 8.1 8.2 8.3 Weber, A.K., Barber, L.B., LeBlanc, D.R., Sunderland, E.M. and Vecitis, C.D., 2017. Geochemical and Hydrologic Factors Controlling Subsurface Transport of Poly- and Perfluoroalkyl Substances, Cape Cod, Massachusetts. Environmental Science and Technology, 51(8), pp. 4269-4279. DOI: 10.1021/acs.est.6b05573 Free Download
- ^ 9.0 9.1 9.2 Anderson, R.H., Adamson, D.T. and Stroo, H.F., 2019. Partitioning of poly-and perfluoroalkyl substances from soil to groundwater within aqueous film-forming foam source zones. Journal of Contaminant Hydrology, 220, pp. 59-65. DOI: 10.1016/j.jconhyd.2018.11.011 Manuscript available from ResearchGate
- ^ Simcik, M.F. and Dorweiler, K.J., 2005. Ratio of Perfluorochemical Concentrations as a Tracer of Atmospheric Deposition to Surface Waters. Environmental Science and Technology, 39(22), pp. 8678-8683. DOI: 10.1021/es0511218 Free download available from ResearchGate
- ^ Prevedouros, K., Cousins, I.T., Buck, R.C. and Korzeniowski, S.H., 2006. Sources, Fate and Transport of Perfluorocarboxylates. Environmental Science and Technology, 40(1), pp. 32-44. DOI: 10.1021/es0512475 Free download available from Academia
- ^ Ahrens, L., Shoeib, M., Harner, T., Lane, D.A., Guo, R. and Reiner, E.J., 2011. Comparison of Annular Diffusion Denuder and High Volume Air Samplers for Measuring Per- and Polyfluoroalkyl Substances in the Atmosphere." Analytical Chemistry, 83(24), pp. 9622-9628. DOI: 10.1021/ac202414w Free download available from Informea.
- ^ Rauert, C., Shoieb, M., Schuster, J.K., Eng, A. and Harner, T., 2018. Atmospheric concentrations and trends of poly-and perfluoroalkyl substances (PFAS) and volatile methyl siloxanes (VMS) over 7 years of sampling in the Global Atmospheric Passive Sampling (GAPS) network. Environmental Pollution, 238, pp. 94-102. DOI: 10.1016/j.envpol.2018.03.017 Open access article available from ScienceDirect Report.pdf
- ^ Fang, X., Wang, Q., Zhao, Z., Tang, J., Tian, C., Yao, Y., Yu, J. and Sun, H., 2018. Distribution and dry deposition of alternative and legacy perfluoroalkyl and polyfluoroalkyl substances in the air above the Bohai and Yellow Seas, China. Atmospheric Environment, 192, pp. 128-135. DOI: 10.1016/j.atmosenv.2018.08.052
- ^ Brandsma, S.H., Koekkoek, J.C., van Velzen, M.J.M. and de Boer, J., 2019. The PFOA substitute GenX detected in the environment near a fluoropolymer manufacturing plant in the Netherlands. Chemosphere, 220, pp. 493-500. DOI: 10.1016/j.chemosphere.2018.12.135 Open access article available from ScienceDirect. Report.pdf
- ^ Barton, C.A., Butler, L.E., Zarzecki, C.J., Flaherty, J. and Kaiser, M., 2006. Characterizing Perfluorooctanoate in Ambient Air near the Fence Line of a Manufacturing Facility: Comparing Modeled and Monitored Values. Journal of the Air and Waste Management Association, 56(1), pp. 48-55. DOI: 10.1080/10473289.2006.10464429 Free access article available from Taylor and Francis Online Report.pdf
- ^ Bossi, R., Vorkamp, K. and Skov, H., 2016. Concentrations of organochlorine pesticides, polybrominated diphenyl ethers and perfluorinated compounds in the atmosphere of North Greenland. Environmental Pollution, 217, pp. 4-10. DOI: 10.1016/j.envpol.2015.12.026
- ^ Ahrens, L., Gerwinski, W., Theobald, N. and Ebinghaus, R., 2010. Sources of polyfluoroalkyl compounds in the North Sea, Baltic Sea and Norwegian Sea: Evidence from their spatial distribution in surface water. Marine Pollution Bulletin, 60(2), pp. 255-260. DOI: 10.1016/j.marpolbul.2009.09.013
- ^ De Silva, A.O., Muir, D.C. and Mabury, S.A., 2009. Distribution of perfluorocarboxylate isomers in select samples from the North American environment. Environmental Toxicology and Chemistry: An International Journal 28(9), pp. 1801-1814. DOI: 10.1897/08-500.1
- ^ Armitage, J.M., 2009. Modeling the global fate and transport of perfluoroalkylated substances (PFAS). Doctoral Dissertation, Institutionen för tillämpad miljövetenskap (ITM), Stockholm University. Report.pdf
- ^ Du, Z., Deng, S., Bei, Y., Huang, Q., Wang, B., Huang, J. and Yu, G., 2014. Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents – A review. Journal of Hazardous Materials, 274, pp. 443-454. DOI: 10.1016/j.jhazmat.2014.04.038
- ^ 22.0 22.1 Guelfo, J.L. and Higgins, C.P., 2013. Subsurface Transport Potential of Perfluoroalkyl Acids at Aqueous Film-Forming Foam (AFFF)-Impacted Sites. Environmental Science and Technology, 47(9), pp. 4164-4171. DOI: 10.1021/es3048043 Doctoral Dissertation
- ^ 23.0 23.1 Zhao, L., Bian, J., Zhang, Y., Zhu, L. and Liu, Z., 2014. Comparison of the sorption behaviors and mechanisms of perfluorosulfonates and perfluorocarboxylic acids on three kinds of clay minerals. Chemosphere, 114, pp. 51-58. DOI: 10.1016/j.chemosphere.2014.03.098 Free download available from ResearchGate.
- ^ 24.0 24.1 Brusseau, M.L., 2019. Estimating the relative magnitudes of adsorption to solid-water and air/oil-water interfaces for per-and poly-fluoroalkyl substances. Environmental Pollution, 254B, p. 113102. DOI: 10.1016/j.envpol.2019.113102
- ^ Li, Y., Oliver, D.P. and Kookana, R.S., 2018. A critical analysis of published data to discern the role of soil and sediment properties in determining sorption of per and polyfluoroalkyl substances (PFASs). Science of the Total Environment, 628, pp. 110-120. DOI: 10.1016/j.scitotenv.2018.01.167
- ^ Higgins, C.P. and Luthy, R.G., 2006. Sorption of Perfluorinated Surfactants on Sediments. Environmental Science and Technology, 40(23), pp. 7251-7256. DOI: 10.1021/es061000n
- ^ Chen, H., Chen, S., Quan, X., Zhao, Y. and Zhao, H., 2009. Sorption of perfluorooctane sulfonate (PFOS) on oil and oil-derived black carbon: Influence of solution pH and [Ca2+]. Chemosphere, 77(10), pp. 1406-1411. DOI: 10.1016/j.chemosphere.2009.09.008
- ^ Pan, G., Jia, C., Zhao, D., You, C., Chen, H. and Jiang, G., 2009. Effect of cationic and anionic surfactants on the sorption and desorption of perfluorooctane sulfonate (PFOS) on natural sediments. Environmental Pollution, 157(1), pp.325-330. DOI: 10.1016/j.envpol.2008.06.035 Free download available from ResearchGate
- ^ Brusseau, M.L., 2019. The Influence of Molecular Structure on the Adsorption of PFAS to Fluid-Fluid Interfaces: Using QSPR to Predict Interfacial Adsorption Coefficients. Water Research, 152, pp. 148-158. DOI: 10.1016/j.watres.2018.12.057 Author’s Manuscript
- ^ Lyu, Y., Brusseau, M.L., Chen, W., Yan, N., Fu, X., and Lin, X., 2018. Adsorption of PFOA at the Air-Water Interface during Transport in Unsaturated Porous Media. Environmental Science and Technology, 52(14), pp. 7745-7753. DOI: 10.1021/acs.est.8b02348 Author’s Manuscript
- ^ 31.0 31.1 Brusseau, M.L., Yan, N., Van Glubt, S., Wang, Y., Chen, W., Lyu, Y., Dungan, B., Carroll, K.C., and Holguin, F.O., 2019. Comprehensive Retention Model for PFAS Transport in Subsurface Systems. Water Research, 148, pp. 41-50. DOI: 10.1016/j.watres.2018.10.035 Author’s Manuscript
- ^ 32.0 32.1 Houtz, E.F., Higgins, C.P., Field, J.A. and Sedlak, D.L., 2013. Persistence of perfluoroalkyl acid precursors in AFFF-impacted groundwater and soil. Environmental Science and Technology, 47(15), pp. 8187-8195. DOI: 10.1021/es4018877 Free download from ReseqarchGate
- ^ 33.0 33.1 33.2 McGuire, M.E., Schaefer, C., Richards, T., Backe, W.J., Field, J.A., Houtz, E., Sedlak, D.L., Guelfo, J.L., Wunsch, A., and Higgins, C.P., 2014. Evidence of Remediation-Induced Alteration of Subsurface Poly- and Perfluoroalkyl Substance Distribution at a Former Firefighter Training Area. Environmental Science and Technology, 48(12) pp. 6644-6652. DOI: 10.1021/es5006187 Manuscript available from Oregon State University
- ^ 34.0 34.1 Anderson, R.H., Long, G.C., Porter, R.C. and Anderson, J.K., 2016. Occurrence of select perfluoroalkyl substances at US Air Force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere, 150, pp. 678-685. DOI: 10.1016/j.chemosphere.2016.01.014
- ^ Martin, J.W., Asher, B.J., Beesoon, S., Benskin, J.P. and Ross, M.S., 2010. PFOS or PreFOS? Are perfluorooctane sulfonate precursors (PreFOS) important determinants of human and environmental perfluorooctane sulfonate (PFOS) exposure? Journal of Environmental Monitoring, 12(11), pp.1979-2004. DOI: 10.1039/C0EM00295J Free download from ResearchGate
- ^ Washington, J.W., Ellington, J.J., Jenkins, T.M. and Yoo, H., 2010. Response to Comments on “Degradability of an Acrylate-Linked, Fluorotelomer Polymer in Soil”. Environmental Science and Technology, 44(2), pp. 849-850. DOI: 10.1021/es902672q Free Download from ACS.
- ^ Gauthier, S.A. and Mabury, S.A., 2005. Aqueous photolysis of 8: 2 fluorotelomer alcohol. Environmental Toxicology and Chemistry, 24(8), pp.1837-1846. DOI: 10.1897/04-591R.1 Free download from ResearchGate.
- ^ Plumlee, M.H., McNeill, K. and Reinhard, M., 2009. Indirect Photolysis of Perfluorochemicals: Hydroxyl Radical-Initiated Oxidation of N-Ethyl Perfluorooctane Sulfonamido Acetate (N-EtFOSAA) and Other Perfluoroalkanesulfonamides. Environmental Science and Technology, 43(10), pp.3662-3668. DOI: 10.1021/es803411w Free download from ResearchGate.
- ^ Arvaniti, O.S. and Stasinakis, A.S., 2015. Review on the occurrence, fate and removal of perfluorinated compounds during wastewater treatment. Science of the Total Environment, 524, pp. 81-92. DOI: 10.1016/j.scitotenv.2015.04.023
- ^ Vedagiri, U.K., Anderson, R.H., Loso, H.M. and Schwach, C.M., 2018. Ambient levels of PFOS and PFOA in multiple environmental media. Remediation Journal, 28(2), pp. 9-51. DOI: 10.1002/rem.21548