Petroleum Hydrocarbons (PHCs)
Petroleum hydrocarbon (PHC) contamination is one of the most common environmental issues encountered by environmental professionals. Environmental pollution caused by releases of petroleum to land, surface water, or the subsurface is of concern because chemicals in PHCs can present a risk to human and environmental receptors if concentrations in environmental media are high enough. A variety of remediation technologies have been developed over the years to reduce the concentrations of petroleum hydrocarbon contaminants in soil and groundwater. However, the complete restoration of sites with petroleum contamination in soils and groundwater is challenging because 1) PHCs in the form of light non-aqueous phase liquids (LNAPLs) can become trapped in soil pores as an immobile, residual phase; and 2) some of the chemical compounds in LNAPL can transfer out of the residual LNAPL and migrate along potential exposure pathways in groundwater, soil, sediment, and air. Fortunately, most PHC constituents can biodegrade either in aerobic or anaerobic environments, making PHC contaminated sites somewhat easier to remediate than typical chlorinated solvents or metals contaminated sites.
Related Article(s):
- Polycyclic Aromatic Hydrocarbons (PAHs)
- Monitored Natural Attenuation (MNA) of Fuels
- Sorption of Organic Contaminants
- Natural Source Zone Depletion (NSZD)
- LNAPL Remediation Technologies (Coming soon)
- NAPL Mobility
- LNAPL Conceptual Site Model (Coming soon)
- Natural Attenuation in Source Zone and Groundwater Plume - Bemidji Crude Oil Spill
CONTRIBUTOR(S): Dr. Bilgen Yuncu
Key Resource(s):
- Characteristics of Dissolved Petroleum Hydrocarbon Plumes[1]
- Evaluating LNAPL Remedial Technologies for Achieving Project Goals[2]
- LNAPL-3: LNAPL Site Management: LCSM Evolution, Decision Process, and Remedial Technologies[3]
- New Developments in LNAPL Site Management[4]
- Managing Risk at LNAPL Sites[5]
Introduction
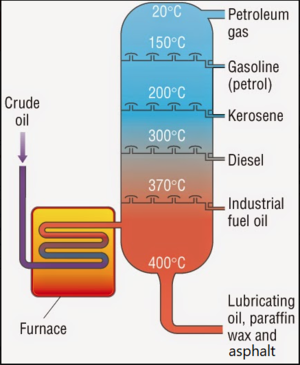
Petroleum hydrocarbons (PHCs) are the primary constituents in crude oil, gasoline (Figure 1), diesel (Figure 2), and a variety of solvents and penetrating oils. Crude Oil consists of hydrocarbon molecules extracted from the ground and transformed in petroleum (oil) refineries into petroleum products, such as gasoline, diesel fuel, asphalt base, heating oil, kerosene, and liquefied petroleum gas. Fractional distillation is used to separate the various compounds of crude oil based on the boiling points of each fraction (Figure 3). The fractions at the bottom of the fractionating column have higher boiling points than at the top. The fractions with higher boiling points are darker in color, more viscous and have less branched alkanes with more carbon atoms and higher molecular weights.
The main classes of PHCs of environmental concern are aromatic hydrocarbons (i.e., benzene, ethylbenzene, toluene, and xylenes), polycyclic aromatic hydrocarbons (PAHs; e.g. anthracene, phenanthrene and benzo[a]pyrene), gasoline additives (e.g., Methyl tert-butyl ether (MTBE), tert-Butyl alcohol (tBA)), and combustion emissions from fuels (e.g., carbon monoxide, acetaldehyde, formaldehyde, and diesel particulates).
Aromatic Hydrocarbons
Aromatic hydrocarbons contain one or more benzene rings. The name of this class comes from the fact that many of them have strong, pungent aromas. Key aromatic hydrocarbons of environmental interest are benzene, ethylbenzene, toluene, and xylenes (BTEX). BTEX compounds are the most common aromatic compounds in petroleum[6]. They are extracted from complex mixtures obtained by the refining of oil or by distillation of coal tar and are used to produce a range of important chemicals, polymers, and consumer products such as paints and lacquers, thinners, rubber products, adhesives, inks, cosmetics and pharmaceutical products. Since BTEX compounds are found naturally in crude oil, coal, and gas deposits, they can be present naturally in groundwater near these deposits.
Benzene is a known human carcinogen and therefore included in risk Group 1 of the International Agency for Research on Cancer (IARC). Long-term exposure to benzene can cause cancer in blood forming organs (i.e. leukemia)[7]. Ethylbenzene is included in risk Group 2B which consists of chemicals considered possibly carcinogenic to humans[8]. Toluene and xylenes are categorized as not classifiable as to human carcinogenicity (Group 3) by both the U.S. Environmental Protection Agency[9][10] and IARC[11], reflecting the lack of evidence for the carcinogenicity of these two chemicals.
Acute (short-term) exposure to BTEX has been associated with skin and sensory irritation, central nervous system problems (tiredness, dizziness, headache, loss of coordination), and effects on the respiratory system (eye and nose irritation). Prolonged (chronic) exposure to BTEX compounds can affect the kidney, liver, and blood systems. Surface water concentrations of BTEX compounds above 1 mg/L can produce acute toxic effects in organisms such as algae and fish.
Polycyclic Aromatic Hydrocarbons
Polycyclic aromatic hydrocarbons (PAHs) are a group of more than 100 different chemicals that are released from burning coal, oil, gasoline, trash, tobacco, wood, or other organic substances such as charcoal-broiled meat. While PAHs occur naturally in crude oil, and smoke and ash from forest fires, they are most often found as products of incomplete combustion, especially from incinerators. PAHs are often found at facilities formerly involved in creosote, coking, and wood preservative production, as well as at former manufactured gas plants that used coal as a feedstock. Most regulations, analyses, and data reporting focus on only a limited number of PAHs, typically between 14 and 20 individual PAH compounds such as: acenaphthene, acenaphthylene, anthracene, benz[a]anthracene, benzo[a]pyrene, benzo[e]pyrene, benzo[b]fluoranthene, benzo[g,h,i]perylene, benzo[j]fluoranthene, benzo[k]fluoranthene, chrysene, dibenz[a,h]anthracene, fluoranthene, fluorene, indeno[1,2,3-c,d]pyrene, naphthalene, phenanthrene and pyrene[12].
Of the more than 100 forms of PAHs, 15 are listed as "reasonably anticipated to be human carcinogens" in the 14th Report on Carcinogens[13] since exposure to these PAHs is linked to lung, liver, and skin cancers. Workers who have been exposed to large amounts of naphthalene from skin contact with the liquid form and from breathing naphthalene vapor have developed blood and liver abnormalities. PAHs are often important contaminants of concern because of their chemical and toxicological properties. Composed of multiple aromatic rings, PAHs tend to be more immobile and more persistent in the environment than the BTEX compounds, with relatively higher bioaccumulation rates. Usually, the two most important PAH compounds at PHC contamination sites are naphthalene, an IARC Group 2B possible carcinogen which is more mobile than other PAHs and typically occurs at higher concentrations, and benzo[a]pyrene, a Group 1 known carcinogen, due to its low threshold of risk to human receptors.
Gasoline (Fuel) Additives
Methyl tert-butyl ether (MTBE) is a gasoline additive used as an oxygenate (raises the oxygen content) to improve combustion of the gasoline and reduce emissions. Although MTBE was effective in reducing emissions from automobile engines, it became a problem when accidently released from leaking underground tanks to groundwater or emitted from two-cycle boat engines to surface water. Key concerns with MTBE releases include the potential to leave an unpleasant taste and odor in water containing MTBE if at high enough concentrations, and potential health risks associated with exposure to MTBE above certain concentration thresholds. MTBE has been identified as a potential human carcinogen by USEPA at high doses[14]. Human exposure to high levels of MTBE in air can also cause irritation of the eyes and respiratory tract, and effects on the central nervous system. Since 2005, the use of MTBE in gasoline has been phased out in the United States. However, groundwater in some areas of the country might still contain MTBE, and it is still being used as a gasoline additive in other parts of the world[14]. Tert-Butyl alcohol (tBA) is another fuel oxygenate, but it is also used in perfumes, as a solvent for pharmaceuticals, and as a paint remover. While MTBE may contain a small percentage of tBA, tBA is also a well‐established biodegradation by-product of MTBE and often found as a groundwater co-contaminant at gasoline-impacted sites[15]. Like MTBE, inhalation of high concentrations of tBA for prolonged exposures may produce transient effects on the central nervous system, as well as eye and mucous membrane irritation[16].
Total Petroleum Hydrocarbons
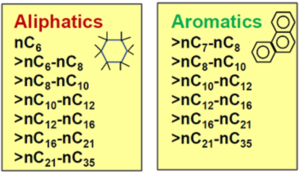
Total petroleum hydrocarbons (TPH) is a term used to describe any mixture of hydrocarbons that are found in crude oil. There are several hundreds of these compounds, but not all occur in any one sample. Because there are so many different chemicals in crude oil and in other petroleum products, it is not practical to measure each one separately. However, it is useful to measure the total amount of petroleum hydrocarbons at a site. TPH can be evaluated in several ways:
- TPH for gasoline range organics (GRO) includes hydrocarbons with 6 to 10 carbon atoms (C6-C10),
- TPH for diesel range organics (DRO) includes hydrocarbons with 10 to 28 carbons (C10-C28), and
- TPH for oil or residual range organics (ORO, RRO) includes hydrocarbons with 28 to 36 carbons (C28-C36).
- Volatile petroleum hydrocarbons (VPH) include C5-C12 aliphatics, BTEX, MTBE, naphthalene, and C9-C10 aromatics.
- Extractable petroleum hydrocarbons (EPH) include C9-C36 aliphatics and C11-C22 aromatics[6].
Because TPH represents a mixture of a large number of chemical compounds, it indicates the amount of petroleum that may be present in a sample but does not provide a direct indication of risk to human health or the environment[18][19]. For example, two soil samples impacted by equal concentrations of either baby oil or gasoline would have virtually identical TPH values, but very different toxicities. The TPH Criteria Working Group (TPHCWG) developed the TPH Fraction Method to calculate risk associated with petroleum hydrocarbon mixtures. The Naval Facilities Engineering Command[4] described the method for applying risk to TPH measurements this way:
With TPHCWG method, TPH is measured using an analytical method that provides somewhat more detail than total TPH concerning the composition of the petroleum mixture. These analytical methods include TCEQ1006 (based on the TPHCWG) or Massachusetts EPH/VPH. These methods provide concentration results for six (Massachusetts EPH/VPH) or 13 (TCEQ1006) different TPH fractions separated into different classes by compound type (aliphatics vs. aromatics, Figure 4) and by carbon number. These fraction results can be used in risk assessments by assigning conservative toxicity values and fate and transport characteristics to each different fraction. The toxicity and fate and transport values can be obtained from guidance documents that describe the application of the TPH fraction approach for risk assessment[20][21]. While typically used to demonstrate that soils containing petroleum hydrocarbons have heaver, low-risk fractions, the method can also be applied to groundwater samples[4].
Physical and Chemical Properties
PHCs are generally divided into two groups: aliphatics and aromatics. Aliphatics include: a) alkanes that contain single bonds between carbon atoms and have formulas of CnH2n+2, b) alkenes, which contain one or more double bonds between carbon atoms and have formulas of CnH2n, and c) cycloalkanes, which contain single-bonded carbon atoms in cyclic structures. Aromatics have one or more benzene rings as part of their structure. Monoaromatics have one benzene ring as part of their structure while polycyclic aromatic hydrocarbons (PAHs) contain two or more bonded benzene rings. Table 1 summarizes the physical and chemical properties of selected PHCs and fuel additives.
| IUPAC Name | Common Name (Acronym) |
Molecular Formula | Chemical Structure | Formula Weight (g/mol) |
Density (g/mL) |
Solubility (mg/L at 20° to 25°C) |
Vapor Pressure (mmHg) |
Henry’s Law Constant (Dimensionless) |
Log Kow[22] | MCL[23] (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| Aromatic Hydrocarbons | ||||||||||
| Benzene | Benzene | C6H6 | 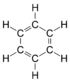 |
7.81 | 0.88 | 1,750[24] | 76[25] | 0.228[24] | 2.13 | 0.005 |
| Ethylbenzene | Ethylbenzene | C8H10 | 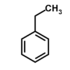 |
106.2 | 0.87 | 169[24] | 7[25] | 0.323[24] | 4.34 | 0.7 |
| Methylbenzene | Toluene | C7H8 | 92.1 | 0.87 | 526[24] | 22[25] | 0.272[24] | 2.73 | 1 | |
| Total Xylenes* | -- | -- | -- | -- | -- | -- | -- | -- | -- | 10 |
| 1,3-Dimethylbenzene | m-Xylene | C8H10 | 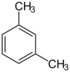 |
106.2 | 0.86 | 161[24] | 6[25] | 0.301[24] | 3.20 | -- |
| 1,2-Dimethylbenzene | o-Xylene | C8H10 | 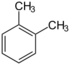 |
106.2 | 0.88 | 178[24] | 5[25] | 0.213[24] | 3.12 | -- |
| 1,4-Dimethylbenzene | p-Xylene | C8H10 | 106.2 | 0.86 | 185[24] | 6.5[25] | 0.314[24] | 3.15 | -- | |
| Polycyclic Aromatic Hydrocarbons | ||||||||||
| 1,2-Dihydroacenaphthylene | Acenaphthene | C12H10 | 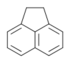 |
154.2 | 1.22 | 4.24[24] | 4.47x10-3[22] | 6.36x10-3[24] | 3.98 | -- |
| Acenaphthylene | Acenaphthylene | C12H8 | 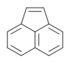 |
152.2 | 0.90 | 3.93[22] | 0.029[22] | 0.0593[22] | 4.07 | -- |
| Anthracene | Anthracene | C14H10 | 178.2 | 1.28 | 0.0434[24] | 1.7x10-5[22] | 2.67x10-3[24] | 4.45 | -- | |
| Benz[a]anthracene | Benz[a]anthracene | C18H12 | 228.3 | 1.27 | 9.4x10-3[24] | 2.2x10-8[22] | 1.4x10-4[24] | 5.61 | -- | |
| Benzo[a]pyrene | Benzo[a]pyrene | C20H12 | 252.3 | 1.35 | 1.6x10-3[24] | 5.6x10-9[22] | 4.63x10-5[24] | 6.06 | 2x10-4 | |
| Benzo[b]fluoranthene | Benzo[b]fluoranthene | C20H12 | 252.3 | 1.29 | 1.5x10-3[24] | 5.0x10-7[22] | 4.55x10-3[24] | 6.04 | -- | |
| Benzo[g,h,i]perylene | Benzo[g,h,i]perylene | C22H12 | 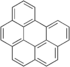 |
276.3 | 1.38 | 2.6x10-4[22] | 1.0x10-10[22] | 5.89x10-6[22] | 6.50 | -- |
| Benzo[k]fluoranthene | Benzo[k]fluoranthene | C20H12 | 252.3 | 1.29 | 8x10-4[24] | 9.6x10-11[22] | 3.4x10-5[24] | 6.06 | -- | |
| Chrysene | Chrysene | C18H12 | 228.3 | 1.27 | 1.6x10-3[24] | 6.3x10-7[22] | 3.88x10-3[24] | 5.16 | -- | |
| Fluoranthene | Fluoranthene | C16H10 | 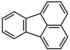 |
202.3 | 1.25 | 0.206[24] | 5.0x10-6[22] | 6.6x10-4[24] | 4.90 | -- |
| 9H-Fluorene | Fluorene | C13H10 | 166.2 | 1.20 | 1.98[24] | 3.2x10-4[22] | 2.61x10-3[24] | 4.18 | -- | |
| Indeno[1,2,3-c,d]pyrene | Indeno[1,2,3-c,d]pyrene | C22H12 | 276.4 | 1.40 | 2.2x10-5[24] | ~10-11to 10-6[22] | 7.0x10-5[24] | 6.58 | -- | |
| Naphthalene | Naphthalene | C10H8 | 128.2 | 1.16 | 31[24] | 0.087[22] | 0.0198[24] | 3.29 | -- | |
| Phenanthrene | Phenanthrene | C14H10 | 178.2 | 1.18 | 1.20[22] | 6.8x10-4[22] | 1.05x10-3[22] | 4.45 | -- | |
| Pyrene | Pyrene | C16H10 | 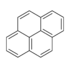 |
202.3 | 1.27 | 0.135[24] | 2.5x10-6[22] | 4.5x10-4[24] | 4.88 | -- |
| Fuel Additives | ||||||||||
| 2-Methoxy-2-methylpropane | Methyl tert-butyl ether (MTBE) | C5H12O | 88.2 | 0.74 | 51,000[26] | 245 to 256[27] | 0.123 to 0.024[27] | 1.20[27] | -- | |
| 2-Methylpropan-2-ol | tert-Butyl alcohol (TBA) | C4H10O | 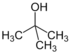 |
74.12 | 0.78 | Infinite[27] | 40 to 42[27] | 4.3x10-4 to 5.9x10-4[27] | 0.35[27] | -- |
| Notes: Kow is the Octanol/Water partition coefficient (dimensionless). MCL is the Maximum Contaminant Level. * Total xylenes consist of three different chemical isomers (meta-xylene, ortho-xylene and para-xylene) but are typically regulated as total xylenes. | ||||||||||
Environmental Concern
Because of the widespread use of petroleum fuels, a large number of the cleanup sites in the United States include PHC contamination. The nature of PHC contamination is highly variable since PHCs themselves are diverse mixtures of chemical components. Several aromatic hydrocarbons such as benzene, naphthalene, and chrysene are known or probable/possible human carcinogens and are classified as priority pollutants by the USEPA[28]. Benzene and PAHs are ranked sixth and ninth, respectively, on the Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) Substance Priority List. This list is a prioritization of substances based on their frequency, toxicity, and potential for human exposure at sites on the National Priorities List (NPL)[22]. Benzene is often the main groundwater contaminant of concern at petroleum release sites because of its higher toxicity and mobility compared to other petroleum hydrocarbons.
Fate and Transport
When petroleum mixtures are released into the environment, the compounds undergo physical, chemical, and biological changes such as volatilization, biodegradation, adsorption onto soil particles, dissolution in water, oxidation, and photodegradation. These processes are collectively referred to as weathering. Fuels are released into the subsurface as undissolved oily-phase liquids that are less dense than water and are therefore commonly referred to as light nonaqueous-phase liquids (LNAPLs). Weathering processes change the distribution of components in the LNAPL. The degree to which various types of petroleum hydrocarbons degrade under these processes depends on the physical and chemical properties of the hydrocarbons and the hydrogeologic and geochemical conditions at the site[21]. A mass balance/modeling study of a 1979 crude oil release[29] provides a detailed look at how long-term weathering processes affected BTEX compounds, short-chained alkanes, long-chained alkanes, and other TPH classes. The model was based in part on a detailed analysis by [30] and was able to replicate the long-term field data that showed toluene, o-xylene, and straight chained alkanes were the compounds that were most depleted over time (see Natural Attenuation in Source Zone and Groundwater Plume - Bemidji Crude Oil Spill).
In general, LNAPLs migrate vertically downward through the soil in the unsaturated zone, and if the release is large enough, that migration may continue until the water table is encountered. The LNAPL will then spread out horizontally along that interface (see NAPL Mobility) with some of the LNAPL above and some below the water table. At some point a continuous LNAPL body will form “residual LNAPL” which can be conceptualized as individual LNAPL blobs in individual pores. As groundwater moves through the mobile and/or residual LNAPL source areas, soluble components partition into the moving groundwater to generate a plume of dissolved contamination. Once further releases stop, these LNAPL source areas tend to slowly weather away as the soluble components, such as BTEX, are depleted[31][32]. A relatively new remediation approach, Natural Source Zone Depletion (NSZD), is based on measuring the rate that the LNAPL in the PHC source zone attenuates. While Monitored Natural Attenuation (MNA) is typically focused on plumes, NSZD focuses on source zones.
The most common sources of fuel components and petroleum hydrocarbons in groundwater are releases of fuels from leaking underground storage tanks (USTs, Figure 5). If gasoline was released from a leaking UST, then the BTEX compounds are often found in soils and in shallow groundwater. BTEX compounds tend to be the most water-soluble fraction of the petroleum compounds and have the lowest soil organic carbon sorption coefficients (Koc, a measure of the tendency of a chemical to bind to soils). Benzene is the most water soluble of the BTEX compounds (10 times more soluble than ethylbenzene or xylenes). BTEX compounds are also the most volatile of the aromatic compounds and are considered to be volatile organic compounds (VOCs) [21].
The BTEX compounds are typically the focus of groundwater cleanups at UST sites. However, because they readily biodegrade under a variety of conditions, BTEX compounds attenuate relatively quickly once they leave the PHC source area, and plumes are relatively short compared to chlorinated solvent plumes. A 1999 meta-analysis of four separate multiple-site studies that analyzed 604 PHC sites found a median PHC plume length of only 132 feet (Figure 6). Two studies evaluated the temporal trends in plume length and found that expanding PHC plumes were rare (less than 8% of plumes) and that most plumes were either stable or shrinking in size.
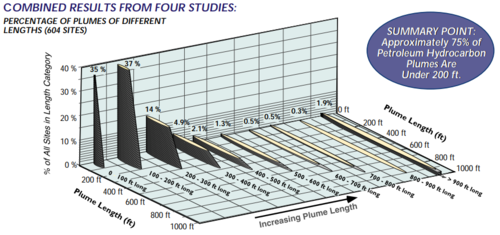
Near the source of gasoline releases that occurred when MTBE was used as a fuel additive, MTBE and tBA are often found commingled with BTEX. Although MTBE and tBA are also classified as VOCs, they tend to have much lower volatility than benzene. Due to their high aqueous solubilities and relatively low retardation factors (i.e. low adsorption to soil particles, low Koc) compared to other fuel components, they were originally expected to form more extensive plumes than BTEX compounds. However, recent studies have shown the opposite to be true due to the fact that MTBE readily degrades under both aerobic and anaerobic conditions in groundwater[33][34].
Components of heavier fuel oils such as complex PAHs like anthracene, phenanthrene, and benzo[a]pyrene more often occur at releases from aboveground storage tanks (ASTs) and oil terminal operations. Most PAHs, because of their low volatility, are classified as semi-volatile organic compounds and are far less soluble in water than other PHC constituents. Since PAHs are generally insoluble in water and have high Koc values, they typically adsorb to sediments and soil and do not form extensive plumes[6].
Recently there has been interest in the presence and behavior of some petroleum metabolites called “polars” because they are polar molecules with a geometric arrangement with one end carrying a net positive charge and the other end a negative charge. This is an emerging field, and researchers are evaluating the toxicity of the metabolites[35] and the characteristic length of the metabolite plumes[36].
Biodegradation is an important weathering and natural attenuation process for petroleum hydrocarbons. The rate of biodegradation depends on the hydrocarbon type, the microbial population present, and the geochemical and hydrological conditions in the subsurface. Because some hydrocarbons are naturally occurring in the environment, populations of bacteria and other organisms capable of degrading petroleum hydrocarbons to a certain extent are ubiquitous in soils and sediments[37][38][39][31]. Generally, petroleum hydrocarbons are good food sources (electron donors) for microorganisms because they contain high-energy electrons with abundant carbon-hydrogen bonds. Biodegradation of PHCs and fuel additives can occur under both aerobic and anaerobic conditions[31][16].
Applicable Remediation Technologies
In cases where mobile LNAPL (free product) removal is feasible, most regulatory agencies require site owners or responsible parties to remove the product (e.g. by pumping or skimming the LNAPL). However, at many sites complete LNAPL removal is not feasible with available technology because residual LNAPL is trapped in soil by strong capillary forces making it difficult to pump out all of the potentially mobile LNAPL. LNAPL removal to the “maximum extent practicable” will, in most cases (except for complete removal by excavation), leave some residual LNAPL behind in the subsurface.
Recent Developments
Because of an increased understanding of the behavior of LNAPL, there has been a movement away from the traditional use of LNAPL thickness in monitoring wells as a metric for assessing the feasibility of LNAPL removal by pumping, favoring instead the use LNAPL transmissivity measurements or estimates to decide whether LNAPL recovery is practicable[2] (see NAPL Mobility for more on LNAPL transmissivity). In addition, there have been significant developments in the use of Natural Source Zone Depletion (NSZD)[40] for managing LNAPL source zones that compliments the historic use of monitored natural attenuation (MNA) of PHC plumes.
PHC Cleanup Technologies
The ITRC[2] has prepared a guidance document that provides a framework for selecting LNAPL Remediation Technologies. Seventeen LNAPL remedial technologies are considered in that guidance document. In 2018 the ITRC updated the guidance to consider a total of 21 remedial technologies[3].
| Soil (Source Zone) | Groundwater (Plume) | |
|---|---|---|
| Ex Situ |
|
|
| In Situ |
|
|
Several common ex situ and in situ remedial technologies used at petroleum-contaminated sites are listed in Table 2[31][2][41].
Ex Situ (Above Ground) Treatment Technologies for Soil
Physical Approaches
- Excavation and off-site disposal: Applicable primarily to unsaturated soils. Requires vertical and lateral access to the contaminated material so that excavation is possible. While often required by regulators where gross contamination has been detected, excavating and treating large amounts of soil is expensive.
- Soil washing (Solvent extraction): Involves the blending of an extraction solution (surfactants, leaching/chelating agents or solvents) with the contaminated soil to extract contaminants from the soil. The solution produced by the washing process is separated from the soil and may be recycled if possible. This technology is rarely used due to high costs associated with disposal of the contaminated wash water.
Thermal Approaches
- Thermal desorption: Applies heat to the excavated contaminated soil to volatilize VOCs and semivolatile organic compounds (SVOCs) and separate them from the soil matrix. As the contaminants vaporize, they can be collected and treated in an off-gas treatment unit.
Biological Approaches
- Land treatment (Land farming): Excavated contaminated soils are placed over a lined treatment area to prevent leaching, or in a biotreatment cell. Volatilization and natural biodegradation are enhanced by tilling (aerating), watering or adding amendments or microorganisms to the soil.
- Slurry biodegradation: Excavated contaminated soil is treated in a slurry solution within a bioreactor vessel that supplies nutrients and microorganisms for biodegradation. The large space and energy requirements for the bioreactors have limited the applicability of this innovative technology.
In Situ Treatment Technologies for Soil/Source Zones
Physical Approaches
-Soil Vapor Extraction (SVE): Applicable to unsaturated soils with moderate to high permeability, SVE relies on a compound’s ability to partition from its adsorbed phase into soil gases which can be vacuum extracted and treated or discharged. The heaver LNAPL constituents may not be volatile enough to be extracted as vapor. SVE provides additional LNAPL removal by increasing subsurface O2 levels and thereby enhancing biodegradation. SVE is one of most frequently used PHC remediation technologies but does not treat LNAPL below the water table.
Thermal Approaches
- Electrical resistance heating (ERH) or Electromagnetic heating: Heating up the soil by inserting electrodes into the soil and passing an alternating current between electrodes (ERH) or by emitting electromagnetic waves at different frequencies (electromagnetic heating) to increase the volatilization of VOCs and SVOCs. Like SVE, volatilized contaminants are then treated at the surface. These methods work best in low permeability soils and can be effective above or below the water table. Because of the large amount of energy required for these methods, they are rarely used at PHC sites.
Chemical Approaches
- In situ soil mixing (ISSM): Involves mixing a chemical treatment agent (e.g. sodium persulfate, calcium peroxide) into the soil by using an auger or tiller system in order to promote chemical oxidation of the contaminant. The capabilities of the mixing equipment may limit the depth of soil to which this method can be applied. This approach may not be practicable in very rocky or consolidated soils.
- Soil Flushing: Involves flushing surfactants or leaching agents through the contaminated area to extract contaminants from the soil. The extraction fluid is then recovered and treated or recycled, if possible. This method can only be applied where the contaminated soil is fairly porous and homogeneous. Because costs associated with treating or disposing large volumes of contaminated extraction fluid can be quite high, this method is rarely used.
Biological Approaches
- Bioventing: Delivering oxygen into the subsurface to stimulate in situ biological activity and aerobic biodegradation of contaminants while minimizing volatilization. If necessary, the activity of indigenous microorganisms is also enhanced by adding nutrients.
- Natural Source Zone Depletion (NSZD): Measuring the degradation rate of an LNAPL body by measuring carbon efflux, thermal heat generation, or other indicators to determine the rate of natural attenuation of source materials. Results are typically reported in gallons per acre per year.
Ex Situ Treatment Technologies for Groundwater
Physical Approaches
- Aggressive fluid vapor recovery (AFVR): Applying dual phase vacuum extraction by using a mobile vacuum truck for quickly removing contaminated groundwater and soil vapors, simultaneously. Contaminated groundwater and soil vapors are treated or disposed offsite.
- Pump and treat (P&T) with discharge, air stripping (AS), carbon adsorption (CA) or advanced oxidation (AO): Applied where hydraulic control of contaminant migration is desirable along with contaminant removal. Discharging contaminated water or treated water to a receiving feature (e.g., stream, wastewater treatment plant) may require a permit. Air stripping may require off-gas treatment. Carbon adsorption cells must be monitored for breakthrough and replacement. Infrequently used due to rapid natural attenuation of dissolved hydrocarbon groundwater plumes.
In Situ Treatment Technologies for Groundwater
Physical Approaches
- Air sparging/oxygen biosparging: Involves inserting sparge wells into the aquifer and pumping pressurized air into groundwater to dissociate the contaminant from aqueous to gas phase. As the contaminated vapor migrates upward into the overlying unsaturated soil, it is then captured by SVE for removal. This is a commonly used technology.
Chemical Approaches
- In situ chemical oxidation (ISCO): involves adding strong chemical oxidants (e.g., sodium persulfate, hydrogen peroxide and Fenton’s reagent, or ozone) to destroy the contaminants in place.
Biological Approaches
- Monitored Natural Attenuation (MNA): relies on naturally occurring microorganisms to degrade the contaminants, leaving behind harmless by-products without outside stimulus. This is the most common approach for managing stable dissolved hydrocarbon plumes.
- In situ bioremediation: Stimulates microbial activity and thus biodegradation by injecting an electron acceptor (e.g., oxygen, nitrate or sulfate). Not commonly used because MNA processes provide sufficient control of many PHC plumes.
References
- ^ 1.0 1.1 Newell, C.J. and Connor, J.A., 1998. Characteristics of dissolved petroleum hydrocarbon plumes, results from four studies. Rapport Technique, American Petroleum Institute, Washington DC. Report.pdf
- ^ 2.0 2.1 2.2 2.3 Interstate Technology and Regulatory Council (ITRC), 2009a. Evaluating LNAPL remedial technologies for achieving project goals. Interstate Technology and Regulatory Council, LNAPLs Team, Washington, DC. Report.pdf
- ^ 3.0 3.1 ITRC, 2018. LNAPL Site Management: LCSM evolution, decision process, and remedial technologies (LNAPL-3). Interstate Technical and Regulatory Council (lnapl-3.itrcweb.org)
- ^ 4.0 4.1 4.2 Naval Facilities Engineering Command (NAVFAC), 2017. Environmental Restoration - New Developments in LNAPL Site Management. ESAT N62583-11-D-0515. Report.pdf
- ^ Sale, T., Hopkins, H. and Kirkman, A., 2018. Managing Risk at LNAPL Sites - Frequently Asked Questions. American Petroleum Institute Tech Bulletin, 18. 72p. Report.pdf
- ^ 6.0 6.1 6.2 Williams, S.D., Ladd, D.E., and Farmer, J.J., 2006. Fate and transport of petroleum hydrocarbons in soil and ground water at Big South Fork National River and Recreation Area, Tennessee and Kentucky, 2002-2003. U.S. Geological Survey Scientific Investigations Report 2005-5104, 29p. Report.pdf
- ^ International Agency for Research on Cancer (IARC), 1982. IARC monographs on the evaluation of carcinogenic risks to humans, some industrial chemicals and dyestuffs, Volume 29. World Health Organization, Lyon, France. ISBN: 978-92-832-1229-4 Report.pdf
- ^ International Agency for Research on Cancer (IARC), 2000. IARC monographs on the evaluation of carcinogenic risks to humans. Volume 77. Report.pdf
- ^ US Environmental Protection Agency (USEPA), 2003. Toxicological review of xylenes, in support of summary information on the integrated risk information system (IRIS), EPA 635/R-03/001. Report.pdf
- ^ US Environmental Protection Agency (USEPA), 2005. Toxicological review of toluene, in support of summary information on the integrated risk information system (IRIS), U.S. Environmental Protection Agency, September Report.pdf
- ^ International Agency for Research on Cancer, 1999. IARC monographs on the evaluation of carcinogenic risks to humans. Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide, 71. Report.pdf
- ^ Agency for Toxic Substance and Disease Registry (ATSDR), 1995. Public health statement for polycyclic aromatic hydrocarbons (PAHs). US Department of Health and Human Services. Report.pdf
- ^ National Toxicology Program (NTP), 2016. Polycyclic Aromatic Hydrocarbons: 15 listings, fourteenth report on carcinogens. U.S. Department of Health and Human Services, Research Triangle Park, NC Report.pdf
- ^ 14.0 14.1 US Environmental Protection Agency (USEPA), 2016. Fuels and fuel additives: Methyl Tertiary Butyl Ether (MTBE). Overview
- ^ Schmidt, T.C., Schirmer, M., Weiß, H. and Haderlein, S.B., 2004. Microbial degradation of methyl tert-butyl ether and tert-butyl alcohol in the subsurface. Journal of contaminant hydrology, 70(3-4), pp.173-203. doi: 10.1016/j.jconhyd.2003.09.001
- ^ 16.0 16.1 Interstate Technology and Regulatory Council, 2005. Overview of groundwater remediation technologies for MTBE and TBA. MTBE-1. Interstate Technology & Regulatory Council, MTBE and Other Fuel Oxygenates Team, Washington, DC. Report.pdf
- ^ Rhodes, 2006. TPH in the Texas Risk Reduction Program (TRRP). Shell Global Solutions Presentation.pdf
- ^ Agency for Toxic Substance and Disease Registry (ATSDR), 1999. Toxicological profile for total petroleum hydrocarbons (TPH). US Department of Health and Human Services. Report.pdf
- ^ American Petroleum Institute (API), 2001. Risk-based methodologies for evaluating petroleum hydrocarbon impacts at oil and natural gas E&P sites. API Publication Number 4709.
- ^ Massachusetts Department of Environmental Protection (MassDEP), M., 2002. Characterizing risks posed by petroleum contaminated sites: implementation of the MADEP VPH/EPH approach. Final Policy# WSC-02-411. Commonwealth of Massachusetts Executive Office of Environmental Affairs. Report.pdf
- ^ 21.0 21.1 21.2 Weisman, W., 1998. Analysis of petroleum hydrocarbons in environmental media, total petroleum hydrocarbon criteria working group series, v.1. Amherst Scientific Publishers, Amherst, Mass. Report.pdf
- ^ 22.00 22.01 22.02 22.03 22.04 22.05 22.06 22.07 22.08 22.09 22.10 22.11 22.12 22.13 22.14 22.15 22.16 22.17 22.18 22.19 22.20 22.21 22.22 Agency for Toxic Substance and Disease Registry (ATSDR), 2019. Priority list of hazardous substances. US Department of Health and Human Services. Substance Priority List Toxic Substances Portal
- ^ US Environmental Protection Agency (USEPA), 2020. National primary drinking water regulations Drinking Water Regulations
- ^ 24.00 24.01 24.02 24.03 24.04 24.05 24.06 24.07 24.08 24.09 24.10 24.11 24.12 24.13 24.14 24.15 24.16 24.17 24.18 24.19 24.20 24.21 24.22 24.23 24.24 24.25 24.26 24.27 24.28 24.29 24.30 24.31 24.32 24.33 24.34 24.35 US Environmental Protection Agency (USEPA), 1996. Soil screening guidance: User’s guide. U.S. Environmental Protection Agency, Office of Solid Waste and Emergency Response, Washington, D.C. Publication 9355.4-23. Publication
- ^ 25.0 25.1 25.2 25.3 25.4 25.5 Howard, P.H., 1989. Handbook of Environmental Fate and Exposure Data for Organic Chemicals, Vol. 1 - Large Production and Priority Pollutants. Lewis Publishers, Inc. Chelsea, Michigan.
- ^ National Center for Biotechnology Information (NCBI), 2020. PubChem Database. Methyl tert-butyl ether, CID=15413. Publication
- ^ 27.0 27.1 27.2 27.3 27.4 27.5 27.6 National Science and Technology Council (NSTC), 1997. Interagency Assessment of Oxygenated Fuels. Committee on Environment and Natural Resources. Report.pdf
- ^ US Environmental Protection Agency (USEPA), 2014. Priority pollutant list. Report.pdf
- ^ Ng, G.H.C., Bekins, B.A., Cozzarelli, I.M., Baedecker, M.J., Bennett, P.C., Amos, R.T. and Herkelrath, W.N., 2015. Reactive transport modeling of geochemical controls on secondary water quality impacts at a crude oil spill site near Bemidji, MN. Water Resources Research, 51(6), pp.4156-4183. Report.pdf
- ^ Baedecker, M.J., Eganhouse, R.P., Bekins, B.A. and Delin, G.N., 2011. Loss of volatile hydrocarbons from an LNAPL oil source. Journal of Contaminant Hydrology, 126(3-4), pp.140-152. doi: 10.1016/j.jconhyd.2011.06.006
- ^ 31.0 31.1 31.2 31.3 Wiedemeier, T.H., Wilson, J.T., Kampbell, D.H., Miller, R.N., and Hansen, J.E., 1999. Technical protocol for implementing intrinsic remediation with long-term monitoring for natural attenuation of fuel contamination dissolved in groundwater, v.1. Air Force Center for Environmental Excellence, Technology Transfer Division, Brooks Air Force Base, San Antonio, Texas Report.pdf
- ^ Parsons Engineering Science, Inc, 1999. Final, Methyl tert-Butyl Ether (MTBE), Its movement and fate in the environment and potential for natural attenuation, Technical Summary Report. Report.pdf
- ^ Kamath, R., Connor, J.A., McHugh, T.E., Nemir, A., Le, M.P. and Ryan, A.J., 2012. Use of long-term monitoring data to evaluate benzene, MTBE, and TBA plume behavior in groundwater at retail gasoline sites. Journal of Environmental Engineering, 138(4), pp.458-469. doi: 10.1061/(ASCE)EE.1943-7870.0000488
- ^ Adamson, D. and Newell, C., 2014. Frequently asked questions about monitored natural attenuation in groundwater. Environmental Security Technology Certification Program (ESTCP), Project ER-201211, Alexandria, VA. Report.pdf
- ^ Zemo, D.A., O'Reilly, K.T., Mohler, R.E., Tiwary, A.K., Magaw, R.I., Synowiec, K.A., 2013. Nature and Estimated Human Toxicity of Polar Metabolite Mixtures in Groundwater Quantified as TPHd/DRO at Biodegrading Fuel Release Sites. Groundwater Monitoring & Remediation, 33(4), pp. 44-56. doi: 10.1111/gwmr.12030
- ^ Bekins, B.A., Cozzarelli, I.M., Erickson, M.L., Steenson, R.A. and Thorn, K.A., 2016. Crude oil metabolites in groundwater at two spill sites. Groundwater, 54(5), pp.681-691. doi: 10.1111/gwat.12419
- ^ Kennedy, L., Everett, J. and Gonzales, J., 2000. Aqueous and mineral intrinsic bioremediation assessment (AMIBA) protocol: Brooks Air Force Base. Tex., Air Force Center for Environmental Excellence, Technology Transfer Division, 286. Report.pdf
- ^ Potter, T.L., and Simmons, K.E., 1998. Composition of petroleum mixtures, Total petroleum hydrocarbon criteria working group series, v.2. Amherst Scientific Publishers, Amherst, Mass. ISBN 1-884-940-19-6
- ^ US Environmental Protection Agency (USEPA), 1999. Use of monitored natural attenuation at superfund, RCRA corrective action, and underground storage tank sites. Report.pdf
- ^ ITRC, 2009b. Evaluating natural source zone depletion at sites with LNAPL. LNAPL-1. Interstate Technology and Regulatory Council, LNAPLs Team, Washington, DC. Report.pdf
- ^ Los Angeles LNAPL Workgroup, 2015. Final report for the LA Basin LNAPL recoverability study. Western States Petroleum Association, Torrance, CA Report.pdf