Phytoplankton (Algae) Blooms
Blooms of phytoplankton or algae can cause major environmental problems. Harmful algal blooms (HABs) occur when phytoplankton (algae and cyanobacteria) rapidly increase or accumulate, producing harmful conditions that negatively impact people, freshwater and marine ecosystems, and economies. Certain environmental conditions including high nutrient concentrations from stormwater runoff or wastewater and insufficient mixing of the water column can trigger HABs.
Related Article(s):
Contributor(s): Dr. Nathan Hall and Dr. Katie van Werkhoven
Key Resource(s):
Phytoplankton
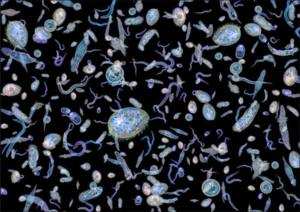
Phytoplankton are microscopic, unicellular, filamentous, or colonial, photosynthetic microalgae or cyanobacteria that live in water (Figure 1). A phytoplankton bloom is the development of a level of phytoplankton biomass that is uncharacteristically high for a given water body[1]. Often, but not always, blooms are formed by a single species.
For a phytoplankton bloom to occur, the net growth rate of a population must be positive for enough time to build a high biomass level. The net growth rate can be described as
- Equation 1: Net Growth = Division – Mortality – Sedimentation – Dilution.
The division rate, also called the intrinsic growth rate, is the rate of new cell production. The total rate of cell loss is driven by three mechanisms: mortality, dilution, and sedimentation.
Phytoplankton Growth Rate. Like terrestrial plants, phytoplankton require sunlight and inorganic nutrients to produce new biomass. Limited supplies of light or nutrients can slow or stop cell division, preventing bloom formation.
Nitrogen and phosphorus are the nutrients most likely to be in short supply relative to demand, and by Liebig’s law of the minimum, are the primary growth-limiting nutrients. Nitrogen supplies tend to be limited in marine waters, and phosphorus supplies tend to be limited in freshwaters. However, many exceptions to these trends exist[2][3]. Humans contribute to nitrogen and phosphorus loads primarily through wastewater inputs and runoff from agricultural, urban, and residential land. While anthropogenic loads increase the probability that a bloom will occur, there must also be sufficient light and low enough loss rates (mortality, sedimentation and dilution) for a bloom to develop.
The amount of light available for phytoplankton growth varies according to time of year, extent of cloud cover, and water depth and clarity. During summer, higher light levels and higher water temperatures promote phytoplankton growth. Additionally, heating of surface waters can create a surface layer that is less dense than cooler bottom waters, separated by a region of strong density gradient called the thermocline. Strong density gradients resist mixing by wind or currents and can confine phytoplankton to a shallow upper mixed layer where there is enough light for phytoplankton growth. In estuaries, salinity differences between upper and lower layers can enhance vertical stratification, creating similar favorable conditions for phytoplankton growth in the upper layer.
Phytoplankton Mortality. Consumption by organisms at higher trophic levels generally constitutes the largest source of mortality for phytoplankton. Grazing by protistan zooplankton is often the dominant source of mortality for phytoplankton in marine waters[4], while grazing by crustacean zooplankton is often more important in freshwaters[5]. In shallow systems with a relatively low ratio of volume to benthic surface area, grazing by benthic bivalves can be substantial and dominate grazing losses[6]. Infections by viruses, fungi, bacteria, and protists can also contribute substantially to phytoplankton mortality. High cell densities of a single-species bloom favor the spread of infections during blooms, and can result in rapid bloom termination[7][8].
Sedimentation. Phytoplankton are typically 3 to 5 percent denser than their surrounding environment. Consequently, most phytoplankton are constantly sinking and require turbulent mixing to stay in the upper mixed layer where light levels are appropriate for growth. According to Stokes’ law, larger and more dense phytoplankton tend to sink faster (0.1-1 m/d) while settling velocities are negligible for the smallest phytoplankton (0.001 m/d)[9]. Many phytoplankton, particularly bloom-forming taxa, avoid settling losses by having either flagella that allow them to swim or ballast mechanisms that provide buoyancy[10]. Cyanobacteria are notorious for accumulating by flotation into dense surface scums (Figure 2).
Dilution. In a water body with constant volume, any water inputs must be accompanied by an equivalent water loss. For example, riverine water inputs to a lake are matched by outflows from the lake. If we assume that the river contains negligible phytoplankton, then river water dilutes phytoplankton densities in the lake at a rate equivalent to the river flow rate divided by lake volume. If the dilution rate is higher than net growth rate, then the physical effect of flushing will prevent bloom development.
The reciprocal of dilution rate is the water residence time which is the average amount of time that a parcel of water spends within a body of water. Generally, water bodies with residence times of a few days or less will not develop phytoplankton blooms. In water bodies with long residence times (e.g. more than a couple of weeks), phytoplankton blooms are more likely to develop. In addition to natural flow conditions, residence time and bloom development can be affected by water control infrastructure[11][12][13]. Dams and other flow manipulating structures (e.g. causeways, pumping stations, locks etc.) can significantly increase the residence time and can therefore greatly increase the potential for phytoplankton bloom development.
Consequences of Phytoplankton Blooms
Phytoplankton blooms threaten the health of aquatic organisms and the health of humans, pets, or livestock that use affected waters for drinking or recreation. High concentrations of phytoplankton during bloom conditions colors and clouds the water limiting the transmission of light in the water column. In shallow systems, light levels along the bottom may become insufficient to support beneficial submerged aquatic vegetation (SAV) that provide habitat, remove nutrients from the water column, and stabilize bottom sediments. Once the SAV is gone, suspension of destabilized sediments causes an increase in turbidity, which in turn often prevents the SAV from returning. The nutrients that were previously consumed by SAV, are consumed by phytoplankton instead, further perpetuating blooms. These feedback mechanisms can trap a water body in this undesirable alternative stable state[14].
Of all the negative impacts of phytoplankton blooms, production of toxins by some bloom-forming species represents the most direct threat to human health. Cyanobacteria and dinoflagellates are the most common toxin producing group of phytoplankton in fresh and marine waters, respectively. Cyanobacteria produce a wide variety of cyanotoxins including hepatotoxic (liver-damaging) microcystins, nodularins, and cylindrospermopsins, neurotoxic (nerve-damaging) saxitoxins and anatoxins, and dermatoxic (skin-damaging) lyngbya toxins[15].
Ingestion of toxins in drinking water and contact during recreation activities are the two most common exposure pathways to humans, pets and livestock. Cyanotoxins and taste and odor compounds produced by cyanobacteria can be removed from drinking water by treatment with activated carbon and/or ozone, increasing treatment costs[16]. Some dinoflagellate blooms also produce the neurotoxin saxitoxin which can bioaccumulate in shellfish and cause paralytic shellfish poisoning in humans or other shellfish eating animals. Red tide dinoflagellate blooms of the genus Karenia produce brevetoxins that kill fish and other marine life and, when aerosolized by wave action, can cause respiratory irritation in humans[17].
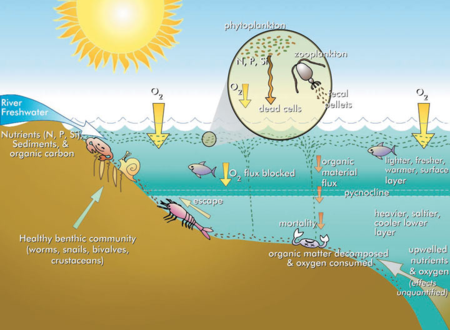
Habitat loss is another potential consequence of phytoplankton blooms. Although phytoplankton photosynthesis produces oxygen, the decomposition of the dead phytoplankton organic matter can deplete dissolved oxygen in the water to levels too low for fish and other animals. The result is restricted habitat availability due to these dead zones and occasionally mass mortality events (i.e., fish kills)[18]. Hypoxia (low oxygen) or anoxia (no oxygen) is particularly common in bottom waters that are disconnected from the atmosphere by a temperature gradient (thermocline) in lakes or salinity gradient ( halocline) in estuaries. Further exacerbating the problem, hypoxia increases nutrient release from sediments. Under hypoxic conditions phosphorus (P) is released from the sediments due to reduction of iron hydroxides that bind phosphate. Additionally, fluxes of ammonium-nitrogen (N) into the water column are enhanced under hypoxic or anoxic conditions when denitrification becomes limited by a lack of nitrate[19]. Under oxic (normal oxygen) conditions, nitrate would be produced by nitrification of ammonium. The increased release of N and P from the sediments under hypoxic conditions can fuel phytoplankton blooms presenting a major challenge to restoring water quality and aquatic habitats. Water bodies that are enriched with nutrients and characterized by degraded habitats are referred to as eutrophic (Figure 3)[20].
Bloom Mitigation Strategies
Bloom mitigation strategies can broadly be grouped by those strategies that address the root causes versus those that alleviate the symptoms of an algal bloom. Elevated nutrient loading and/or manipulation of circulation are common root causes of bloom problems that can be corrected through well designed mitigation strategies.
Reduce External Nutrient Loading. The first step in designing nutrient controls is determining what nutrient(s) limit phytoplankton growth. Nitrogen (N) and phosphorus (P) have been commonly assumed to be the limiting nutrients for fresh and marine waters[2], respectively. However, recent studies[21][22] have shown that the limiting nutrient can change seasonally. Once the limiting nutrients have been identified, nutrient reduction targets are generally formulated using models that relate nutrient loads to phytoplankton biomass. These models may be experimental models with natural water containing the natural phytoplankton communities, simple nutrient budget-based models, or mechanistic, coupled circulation/water quality models. Mechanistic circulation/water quality models are the primary means of designing mitigation options for large water bodies. Enacting watershed-based controls on nutrient sources is the best strategy for large water bodies[23].
Water Column Mixing. Artificial vertical mixing can reduce the intensity of cyanobacteria blooms through two mechanisms. First, mixing oxygenated surface waters downward reduces sediment loading of N and P that results when sediments become anoxic. Second, vigorous vertical mixing negates the floating ability of cyanobacteria which leads to lower light availability and minimizes the competitive advantage buoyant taxa have over more desirable, negatively buoyant taxa (e.g. green algae and diatoms)[24]. Energy requirements to produce sufficiently vigorous mixing are high, and attempts to mix large water bodies with low powered mixers have been unsuccessful[25][26]. For large water bodies, nutrient control and, where possible, prevention of long residence time conditions are the most feasible, long term solutions to bloom problems[23].
Legacy Nutrient Removal. Organic-rich sediments that result from decades of nutrient over-enrichment can continue to provide high internal nutrient loads that fuel blooms even after external sources of N and P have been reduced[23]. Application of alum or modified clay has been used successfully in small to medium sized freshwater bodies to flocculate P and phytoplankton cells out of the water column. Once the clay has settled, it can form a cap on the sediments to prevent P from diffusing back to the water column during anoxic periods. Physical removal of organic rich surficial sediments by dredging has also been used effectively but the high cost of both clay application and dredging largely restricts these practices to small water bodies[23]. Nutrients in a water body can also be intercepted and removed by intentionally growing and harvesting macroalgae in a relatively new process called algal turf scrubbing [27].
Biological and Chemical Controls. For some water bodies, blooms can be managed by manipulating the food web (e.g. removing certain fishes) to increase the numbers of zooplankton grazers[28][29]. As a very short-term fix, algaecides can be used to control phytoplankton blooms. Copper-based algaecides can effectively kill most phytoplankton groups, and algaecides containing hydrogen peroxide can be equally effective on cyanobacteria[30], without potential unintended toxic effects on higher trophic levels[31]. High costs of algaecide also largely restrict its use to small water bodies.
References
- ^ Carstensen, J., Henriksen, P. and Heiskanen, A.S., 2007. Summer algal blooms in shallow estuaries: definition, mechanisms, and link to eutrophication. Limnology and Oceanography, 52(1), pp.370-384. DOI:10.4319/lo.2007.52.1.0370
- ^ 2.0 2.1 Howarth, R.W., 1988. Nutrient limitation of net primary production in marine ecosystems. Annual review of ecology and systematics, 19(1), pp.89-110. doi: 10.1146/annurev.ecolsys.19.1.89
- ^ Sterner, R.W., 2008. On the phosphorus limitation paradigm for lakes. International Review of Hydrobiology, 93(4‐5), pp.433-445. doi: 10.1002/iroh.200811068
- ^ Schmoker, C., Hernández-León, S. and Calbet, A., 2013. Microzooplankton grazing in the oceans: impacts, data variability, knowledge gaps and future directions. Journal of Plankton Research, 35(4), pp.691-706. doi: 10.1093/plankt/fbt023
- ^ Tessier, A.J., Bizina, E.V. and Geedey, K.C., 2001. Grazer - resource interactions in the plankton: Are all daphniids alike. Limnology and Oceanography, 46(7), pp.1585-1595. doi: 10.4319/lo.2001.46.7.1585
- ^ Cloern, J.E., 1982. Does the Benthos Control Phytoplankton Biomass in South San Francisco Bay. Marine Ecology Progress Series. Oldendorf, 9(2), pp.191-202. doi: 10.3354/meps009191
- ^ Lehahn, Y., Koren, I., Schatz, D., Frada, M., Sheyn, U., Boss, E., Efrati, S., Rudich, Y., Trainic, M., Sharoni, S. and Laber, C., 2014. Decoupling physical from biological processes to assess the impact of viruses on a mesoscale algal bloom. Current Biology, 24(17), pp.2041-2046. doi: 10.1016/j.cub.2014.07.046
- ^ Von Donk, E. and Ringelberg, J., 1983. The effect of fungal parasitism on the succession of diatoms in Lake Maarsseveen I (The Netherlands). Freshwater Biology, 13(3), pp.241-251. doi: 10.1111/j.1365-2427.1983.tb00674.x
- ^ Fogg, G.E., 1991. The phytoplanktonic ways of life. New Phytologist, 118(2), pp.191-232. doi: 10.1111/j.1469-8137.1991.tb00974.x
- ^ Paerl, H.W., 1988. Nuisance phytoplankton blooms in coastal, estuarine, and inland waters 1. Limnology and Oceanography, 33(4part2), pp.823-843. doi: 10.4319/lo.1988.33.4part2.0823
- ^ Gasith, A. and Gafny, S., 1998. Importance of Physical Structures in Lakes: the Case of Lake Kinneret and General Implications. In: The Structuring Role of Submerged Macrophytes in Lakes (ed. E. Jeppesen, M. Sondergaard, M. Sondergaard & K. Christoffersen). Ecological Studies (Analysis and Synthesis), vol. 131, pp. 331-338. Springer, New York, NY. doi:10.1007/978-1-4612-0695-8_24
- ^ Hall, N.S., Paerl, H.W., Peierls, B.L., Whipple, A.C. and Rossignol, K.L., 2013. Effects of climatic variability on phytoplankton community structure and bloom development in the eutrophic, microtidal, New River Estuary, North Carolina, USA. Estuarine, Coastal and Shelf Science, 117, pp.70-82. doi: 10.1016/j.ecss.2012.10.004
- ^ Scavia, D. and Liu, Y., 2009. Exploring estuarine nutrient susceptibility. Environmental Science & Technology, 43(10), pp.3474-3479. doi: 10.1021/es803401y
- ^ Scheffer, M., Jeppesen, E. 1998. Alternative Stable States. In: The Structuring Role of Submerged Macrophytes in Lakes (ed. E. Jeppesen, M. Sondergaard, M. Sondergaard & K. Christoffersen). Ecological Studies (Analysis and Synthesis), vol. 131, pp. 397-406. Springer, New York, NY doi: 10.1007/978-1-4612-0695-8_31
- ^ Humpage, A. 2008. Toxin types, toxicokinetics and toxicodynamics. In: Hudnell, K. (Ed). Cyanobacterial harmful algal blooms: State of the science and research needs. Advances in Experimental Medicine and Biology Volume 619, pp 383-416, Springer. doi: 10.1007/978-0-387-75865-7_16
- ^ He, X., Liu, Y.L., Conklin, A., Westrick, J., Weavers, L.K., Dionysiou, D.D., Lenhart, J.J., Mouser, P.J., Szlag, D. and Walker, H.W., 2016. Toxic cyanobacteria and drinking water: impacts, detection, and treatment. Harmful Algae, 54, pp.174-193. doi: 10.1016/j.hal.2016.01.001
- ^ Fleming, L.E., Kirkpatrick, B., Backer, L.C., Walsh, C.J., Nierenberg, K., Clark, J., Reich, A., Hollenbeck, J., Benson, J., Cheng, Y.S. and Naar, J., 2011. Review of Florida red tide and human health effects. Harmful Algae, 10(2), pp.224-233. doi: 10.1016/j.hal.2010.08.006
- ^ Eby, L.A. and Crowder, L.B., 2002. Hypoxia-based habitat compression in the Neuse River Estuary: context-dependent shifts in behavioral avoidance thresholds. Canadian Journal of Fisheries and Aquatic Sciences, 59(6), pp.952-965. doi: 10.1139/f02-067
- ^ Kemp, W.M., Sampou, P., Caffrey, J., Mayer, M., Henriksen, K. and Boynton, W.R., 1990. Ammonium recycling versus denitrification in Chesapeake Bay sediments. Limnology and Oceanography, 35(7), pp.1545-1563. doi: 10.4319/lo.1990.35.7.1545
- ^ Bricker, S.B., Clement, C.G., Pirhalla, D.E., Orlando, S.P., Farrow, D.R.G. 1999. National estuarine eutrophication assessment: effects of nutrient enrichment in the nation's estuaries. Silver Spring: NOAA, National Ocean Service, Special Projects Office and the National Centers for Coastal Ocean Science. Report.pdf
- ^ Lewis Jr, W.M. and Wurtsbaugh, W.A., 2008. Control of lacustrine phytoplankton by nutrients: erosion of the phosphorus paradigm. International Review of Hydrobiology, 93(4‐5), pp.446-465. doi: 10.1002/iroh.200811065
- ^ Paerl, H.W., Scott, J.T., McCarthy, M.J., Newell, S.E., Gardner, W.S., Havens, K.E., Hoffman, D.K., Wilhelm, S.W. and Wurtsbaugh, W.A., 2016. It takes two to tango: When and where dual nutrient (N & P) reductions are needed to protect lakes and downstream ecosystems. Environmental Science & Technology, 50(20), pp.10805-10813. doi: 10.1021/acs.est.6b02575
- ^ 23.0 23.1 23.2 23.3 Paerl, H.W., Hall, N.S. and Calandrino, E.S., 2011. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Science of the Total Environment, 409(10), pp.1739-1745. doi: 10.1016/j.scitotenv.2011.02.001
- ^ Huisman, J., Sharples, J., Stroom, J.M., Visser, P.M., Kardinaal, W.E.A., Verspagen, J.M. and Sommeijer, B., 2004. Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology, 85(11), pp.2960-2970. doi: 10.1890/03-0763
- ^ Olson, I., 2016. Evaluating the effectiveness of water remediation techniques for nutrient reduction and the control of cyanobacteria blooms in municipal drinking water reservoirs in the SE United States. Master's Thesis, N.C. State University. Report.pdf
- ^ Upadhyay, S., Bierlein, K.A., Little, J.C., Burch, M.D., Elam, K.P. and Brookes, J.D., 2013. Mixing potential of a surface-mounted solar-powered water mixer (SWM) for controlling cyanobacterial blooms. Ecological Engineering, 61, pp.245-250. doi: 10.1016/j.ecoleng.2013.09.032
- ^ Craggs, R.J., Adey, W.H., Jenson, K.R., St. John, M.S., Green, F.B. and Oswald, W.J., 1996. Phosphorus removal from wastewater using an algal turf scrubber. Water Science and Technology, 33(7), pp.191-198. doi: 10.2166/wst.1996.0138
- ^ Carpenter, S.R., Kitchell, J.F. and Hodgson, J.R., 1985. Cascading trophic interactions and lake productivity. BioScience, 35(10), pp.634-639. doi: 10.2307/1309989
- ^ Triest, L., Stiers, I. and Van Onsem, S., 2016. Biomanipulation as a nature-based solution to reduce cyanobacterial blooms. Aquatic Ecology, 50(3), pp.461-483. doi: 10.1007/s10452-015-9548-x
- ^ Matthijs, H.C., Visser, P.M., Reeze, B., Meeuse, J., Slot, P.C., Wijn, G., Talens, R. and Huisman, J., 2012. Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Research, 46(5), pp.1460-1472. doi: 10.1016/j.watres.2011.11.016
- ^ Willis, B.E. and Bishop, W.M., 2016. Understanding fate and effects of copper pesticides in aquatic systems. Journal of Geoscience and Environment Protection, 4(05), pp. 37 - 42. doi: 10.4236/gep.2016.45004