|
|
| (90 intermediate revisions by the same user not shown) |
| Line 1: |
Line 1: |
| − | '''Munitions Constituents – Photolysis'''
| + | Climate change affects both terrestrial<ref>Diffenbaugh, N. S. and Field, C. B., 2013. Changes in Ecologically Critical Terrestrial Climate Conditions. Science, 341(6145), pp. 486-492. [https://doi.org/10.1126/science.1237123 doi: 10.1126/science.1237123]</ref> and aquatic biomes<ref>Hoegh-Guldberg, O., and Bruno, J. F., 2010. The Impact of Climate Change on the World’s Marine Ecosystem. Science, 328(5985), pp. 1523-1528. [https://doi.org/10.1126/science.1189930 doi: 10.1126/science.1189930]</ref> causing significant effects on ecosystem functions and biodiversity<ref name=":0">Bellard, C., Berteslsmeier, C., Leadley, P., Thuiller, W., and Courchamp, F., 2012. Impacts of climate change on the future of biodiversity. Ecological Letters, 15(4), pp. 365-377. [https://doi.org/10.1111/j.1461-0248.2011.01736.x doi: 10.1111/j.1461-0248.2011.01736.x] [//www.enviro.wiki/images/a/a4/Bellard2012.pdf Article pdf]</ref>. Climate change is affecting several key ecological processes and patterns that will have cascading impacts on wildlife and habitat<ref name=":1">Inkley, D. B., Anderson, M. G., Blaustein, A. R., Burkett, V. R., Felzer, B., Griffith, B., Price, J., and Root, T. L., 2004. Global Climate Change and Wildlife in North America. Wildlife Society Technical Review 04-2. The Wildlife Society, Bethesda, MD, 26 pp. [//www.enviro.wiki/images/f/f1/Inkley2004.pdf Report pdf]</ref>. For example, sea-level rise, changes in the timing and duration of growing seasons, and changes in primary production are mainly driven by changes to global environmental variables (e.g., temperature and atmospheric CO<sub>2</sub>). Climate-induced changes in the environment ultimately impact wildlife population abundance and distributions. |
| − | | + | <br /> |
| − | Munitions compounds (MCs), including [[wikipedia:TNT|2,4,6-trinitrotoluene]] (TNT), [[wikipedia:RDX|hexahydro-1,3,5-trinitro-1,3,5-triazine]] (RDX), [[wikipedia:2,4-Dinitroanisole|2,4-dinitroanisole]] (DNAN), 3-nitro-1,2,4-triazol-5-one (NTO), and [[wikipedia:Nitroguanidine|nitroguanidine]] (NQ), absorb light in the [[wikipedia:Ultraviolet|ultraviolet]] (UV) range and are therefore susceptible to photolysis on soil surfaces and in surface water. Photochemical reactions are important to consider when assessing the environmental impact of MCs since they can yield products that differ from their parent compounds in both toxicity and transport behavior. Quantum yield calculations can aid in predicting the photolysis rates and half-lives of MCs. The photolysis of MCs may be enhanced or inhibited in the presence of compounds that are also excited by UV irradiation.
| + | <div style="float:right;margin:0 0 2em 2em;">__TOC__</div> |
| − | | |
| − | <br /><div style="float:right;margin:0 0 2em 2em;">__TOC__</div> | |
| | | | |
| | '''Related Article(s):''' | | '''Related Article(s):''' |
| | | | |
| − | *[[Munitions Constituents]] | + | *[[Climate Change Primer|Climate Change]] |
| − | *[[Munitions Constituents - Alkaline Degradation]] | + | *[[Predicting Species Responses to Climate Change with Population Models]] |
| − | *[[Munitions Constituents - Composting]]
| |
| − | *[[Munitions Constituents - Deposition]]
| |
| − | *[[Munitions Constituents - Dissolution]]
| |
| − | *[[Munitions Constituents - Sorption]]
| |
| | | | |
| | | | |
| − | '''Contributor(s)''': Dr. Warren Kadoya | + | '''Contributor(s):''' Dr. Breanna F. Powers and Dr. Julie A. Heath |
| | | | |
| | | | |
| | '''Key Resource(s):''' | | '''Key Resource(s):''' |
| | | | |
| − | *Environmental Organic Chemistry, Chapter 15: Direct Photolysis<ref name=":1">Schwarzenbach, R.P., Gschwend, P.M., and Imboden, D.M., 2003. Chapter 15 - Direct Photolysis. <u>In</u>: R.P. Schwarzenbach, P.M. Gschwend, and D.M. Imboden (eds). Environmental Organic Chemistry. 2nd ed. John Wiley & Sons, Inc, Hoboken, NJ, pp. 611-654. ISBN: 9780471350538/eISBN: 9780471649649 [https://doi.org/10.1002/0471649643.ch15 doi:10.1002/0471649643.ch15]</ref> | + | *Global climate change and wildlife in North America. Wildlife Society Technical Review 04-2<ref name=":1" /> |
| − | *[//www.enviro.wiki/images/6/64/ERDC2007.pdf Photochemical Degradation of Composition B and Its Components]<ref name=":2">Pennington, J.C., Thorn, K.A., Co, L.G., MacMillan, D.K., Yost, S., and Laubscher, R.D., 2007. Photochemical Degradation of Composition B and Its Components. U.S. Army Engineer Research and Development Center (ERDC)/ Environmental Laboratory (EL) [https://hdl.handle.net/11681/6837 TR-07-16]. [//www.enviro.wiki/images/6/64/ERDC2007.pdf Report pdf]</ref>
| + | *Impacts of climate change on the future of biodiversity<ref name=":0" /> |
| − | *Verification of RDX Photolysis Mechanism<ref name=":6">Peyton, G.R., LeFaivre, M.H., and Maloney, S.W., 1999. Verification of RDX photolysis mechanism. U.S. Army Engineer Research and Development Center (ERDC)/ Construction Engineering Research Laboratory (CERL) [https://apps.dtic.mil/sti/citations/ADA371755 TR 99/93]. [//www.enviro.wiki/images/c/ca/ADA1999.pdf Report pdf]</ref>
| |
| − | *Photochemical transformation of the insensitive munitions compound 2,4-dinitroanisole<ref name=":9">Rao, B., Wang, W., Cai, Q., Anderson, T., and Gu, B., 2013. Photochemical Transformation of The Insensitive Munitions Compound 2,4-Dinitroanisole. Science of The Total Environment, 443, pp. 692-699. [https://doi.org/10.1016/j.scitotenv.2012.11.033 doi: 10.1016/j.scitotenv.2012.11.033]</ref> | |
| − | *Photo-transformation of aqueous nitroguanidine and 3-nitro-1,2,4-triazol-5-one: Emerging munitions compounds<ref name=":12">Becher, J.B., Beal, S.A., Taylor, S., Dontsova, K., Wilcox, D.E., 2019. Photo-transformation of aqueous nitroguanidine and 3-nitro-1,2,4-triazol-5-one: Emerging munitions compounds. Chemosphere, 228, pp. 418-426. [https://doi.org/10.1016/j.chemosphere.2019.04.131 doi:10.1016/j.chemosphere.2019.04.131]</ref>
| |
| | | | |
| | ==Introduction== | | ==Introduction== |
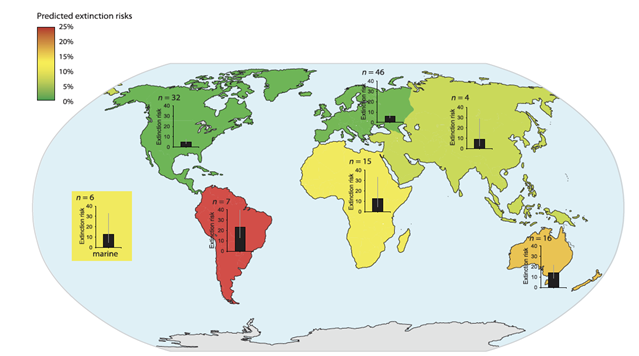
| − | [[File: PhotolysisFig1.png | thumb | 500px | left | Figure 1. Color change over time as particles of IMX-101 and IMX-104 were exposed to sunlight and rainfall, suggesting phototransformation. Image courtesy of the U.S. Army Engineer Research and Development Center<ref name=":15">Dontsova, K., Taylor S., Pesce-Rodriguez, R., Brusseau, M., Arthur, J., Mark, N., Walsh, M., Lever, J., and Simunek, J., 2014. Dissolution of NTO, DNAN, and insensitive munitions formulations and their fates in soils. U.S. Army Engineer Research and Development Center (ERDC)/ Cold Region Research and Engineering Laboratory (CRREL) [https://apps.dtic.mil/sti/citations/ADA609594 TR-14-23]. [[Special:FilePath/Dontsova-2014-Dissolution_and_Fate.pdf| Report pdf]] | + | [[File:PowersFig1.png|thumb|900px|left | Figure 1. The predicted extinction risk (by percentage with 95% CIs) from climate change by different regions, colors represent a gradient from least to most extinction risks (green to red) based upon the number of relevant studies (n). Figure is from Urban (2015)<ref name=":2" />. Reprinted with permission from AAAS. Any use of this figure requires the prior written permission of AAAS]] |
| − | </ref>.]][[wikipedia:Insensitive_munition|Insensitive munitions]], including [[wikipedia:IMX-101|IMX-101]] and IMX-104, are replacing traditional explosives because they are less prone to accidental detonation and therefore safer for military personnel to handle. IMX-101, composed of [[wikipedia:2,4-Dinitroanisole|2,4-dinitroanisole]] (DNAN), 3-nitro-1,2,4-triazol-5-one (NTO), and [[wikipedia:Nitroguanidine|nitroguanidine]] (NQ), will replace [[wikipedia:TNT|2,4,6-trinitrotoluene]] (TNT) in artillery; IMX-104, composed of DNAN, NTO, and [[wikipedia:RDX|hexahydro-1,3,5-trinitro-1,3,5-triazine]] (RDX), will replace [[wikipedia:Composition_B|Composition B]] (Comp B) in mortars<ref>BAE Systems, 2021. [https://www.baesystems.com/en-us/feature/making-explosives-safer. Making explosives safer]</ref>. As both traditional munitions compounds and these insensitive munitions compounds (collectively referred to as MCs) may be deposited onto firing ranges via incomplete detonation, understanding their environmental fate is of concern<ref>Pennington, J.C., Silverblatt, B., Poe, K., Hayes, C.A., and Yost, S, 2008. Explosive residues from low-order detonations of heavy artillery and mortar rounds. Soil and Sediment Contamination: An International Journal, 17(5), pp. 533-546. [https://doi.org/10.1080/15320380802306669 doi: 10.1080/15320380802306669]</ref>. Phototransformation due to sunlight exposure is an important fate-controlling parameter for MCs and can occur on the surfaces of solid explosive particles, as shown in Figure 1, as well as in the aqueous phase following MC dissolution by rainwater. Furthermore, MC photolysis can be affected by the presence of natural organic matter and other compounds that are excited by sunlight.
| + | Global climate change will affect ecosystem functions and cycles such as nutrient, hydraulic, and carbon cycles, changing aspects of environmental conditions such as temperature, soil moisture, and precipitation<ref>Davidson, E. A. and Janssens, I. A., 2006. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature, 440, pp. 165-173.[https://doi.org/10.1038/nature04514 doi: 10.1038/nature04514]</ref><ref>Melillo, J. M., McGuire, A. D., Kicklighter, D. W., Moore, B., Vorosmarty, C. J., and Schloss, A. L., 1993. Global climate change and terrestrial net primary production. Nature, 363, pp. 234-240. [https://doi.org/10.1038/363234a0 doi: 10.1038/363234a0]</ref>. Wildlife species are adapted to their environments and changes to the environment and habitat conditions will mediate effects, either directly or indirectly, on species survival, fecundity and ultimately population persistence<ref>Alig, R. J., Technical Coordinator, 2011. Effects of Climate Change on Natural Resources and Communities: A Compendium of Briefing Papers. U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station, General Technical Report, [https://www.fs.usda.gov/treesearch/pubs/37513 PNW-GTR-837], Portland, OR, 169p. [https://doi.org/10.2737/PNW-GTR-837 doi:10.2737/PNW-GTR-837] [//www.enviro.wiki/images/f/f2/Pnwgtr837.pdf Report pdf]</ref><ref>Acevedo-Whitehouse, K., and Duffus, A. L. J., 2009. Effects of environmental change on wildlife health. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1534), pp. 3429-3438. [https://doi.org/10.1098/rstb.2009.0128 doi: 10.1098/rstb.2009.0128]</ref><ref>Milligan, S. R., Holt, W. V., and Lloyd, R., 2009. Impacts of climate change and environmental factors on reproduction and development in wildlife. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1534), pp. 3313-3319. [https://doi.org/10.1098/rstb.2009.0175 doi: 10.1098/rstb.2009.0175] [//www.enviro.wiki/images/a/ad/Milligan2009.pdf Article pdf]</ref>. The ability to adapt to changing habitat conditions as a result of climate change will differ across individual species and between populations. Some wildlife species may be more vulnerable to climate change than other species (Figure 1). Vulnerability is often linked to particular life-history traits (e.g., specialized habitat needs or limited dispersal abilities, see Pacifici et al. 2015P<ref>Pacifici, M., Foden, W., and Visconti, P., 2015. Assessing species vulnerability to climate change. Nature Climate Change 5, pp. 215-225. [https://doi.org/10.1038/nclimate2448 doi: 10.1038/nclimate2448]</ref> for a review on species vulnerability to climate change) or genetic composition. For example, grassland birds may be more vulnerable to changing climate than forest birds as forests can buffer change more so than grasslands<ref>Jarzyna, M. A., Zuckerberg, B., Finley, A. O., and Porter, W. F., 2016. Synergistic effects of climate change and land cover: grassland birds are more vulnerable to climate change. Landscape Ecology, 31(10), pp. 2275-2290. [https://doi.org/10.1007/s10980-016-0399-1 doi: 10.1007/s10980-016-0399-1]</ref>. Projected changes in the climate will generally have adverse effects of wildlife populations<ref>IPCC, 2001. Climate Change 2001: Synthesis Report. A Contribution of Working Groups I, II, and III to the Third Assessment Report of the Intergovernmental Panel on Climate Change. R.T. Watson and the Core Writing Team (eds.). Cambridge University Press, Cambridge, United Kingdom, and New York, NY, USA, 398p. [//www.enviro.wiki/images/5/56/IPCC2001.pdf Report pdf]</ref>, though there are some species coping with climate change or benefitting from environmental change. For example, American kestrels (Falco sparverius) have shifted their breeding phenology to earlier in the year and may now raise two broods of young within a breeding season<ref>Smith, S. H., Steenhof, K., McClure, C. J. W., and Heath, J. A. 2017. Earlier nesting by generalist predatory bird is associated with human responses to climate change. Journal of Animal Ecology, 86(1), pp. 98-107. [https://doi.org/10.1111/1365-2656.12604 doi: 10.1111/1365-2656.12604] [//www.enviro.wiki/images/5/5d/Smith2017.pdf Article pdf]</ref>. |
| − | ==Direct Photolysis==
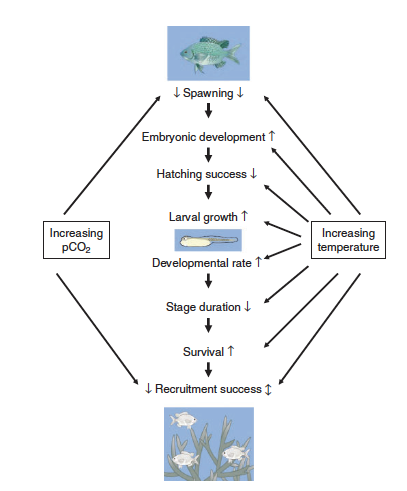
| + | [[File:PowersFig2.png|thumb|900px|right| Figure 2. Conceptual diagram showing how increased water temperatures and pCO₂ (partial pressure of carbon dioxide) affect the early life stages of fish. Where arrow direction indicated increasing rate ( ↑ ), decreasing rate ( ↓ ), or both directions depending on other environmental variables ( ↕️ ). Figure is from Pankhurst and Munday (2011)<ref name=":4" />.]] |
| − | [[File: PhotolysisFig3.png | thumb |530x530px| Figure 3. UV-Vis absorbance spectra for munitions compounds. Spectra over the wavelength range of 200-800 nm were obtained using a Jasco V-630 UV-Vis Spectrophotometer and quartz cuvettes, UV transparent to 200 nm (YeHui Instruments). Adapted from Taylor et al. (2017)<ref>Taylor, S., Becher, J., Beal, S., Ringelberg, D., Spanggord, R., and Dontsova, K., 2017. Photo-transformation of explosives and their constituents. <u>In</u>: The Environmental Aspects of Munitions Workshop, Joint Army-Navy-NASA-Air Force (JANNAF), Kansas City, MO, May 22, 2017.</ref>.]][[File: PhotolysisFig2.png | thumb |377x377px| Figure 2. The ultraviolet and visible spectrum of sunlight measured at noon in midsummer in Cleveland, Ohio in June 1986. Reproduced with permission from [https://www.q-lab.com/ Q-Lab Corporation]<ref name=":0" />.|left]] Compounds that absorb [[wikipedia:Ultraviolet|ultraviolet]] (UV, 100-400 nm) and/or [[wikipedia:Visible_spectrum|visible]] (Vis, 400-800 nm) light can undergo direct photolysis from sunlight. Light is absorbed in discrete units, or [[wikipedia:Photon|photons]], and the energy of these photons is indirectly proportional to the wavelength of the light. When a chemical molecule absorbs a photon, its ground state electrons may become excited. As the excited electrons return to the ground state, they may undergo a chemical reaction that results in transformation. Figure 2 shows that the UV range is further divided into UV-A (315-400 nm), UV-B (280-315 nm), and UV-C (100-280 nm). Because most of UV-C is filtered out by the Earth’s atmosphere, direct photolysis of compounds at the surface primarily involves UV-A and UV-B<ref name=":0">Brennan, P., and Fedor, C., 1994. Sunlight, UV, & accelerated weathering. [https://www.q-lab.com/ Q-Lab Corporation], Technical Bulletin LU-0822. [[Special:FilePath/Brennan1994.pdf| Paper pdf]]</ref>.
| + | ==The influence of climate change on wildlife and habitats== |
| − | The MCs TNT, RDX, DNAN, NTO, and NQ may undergo direct photolysis since they all absorb light in the UV-Vis range (Figure 3). These graphs convey the probability that the compounds will absorb light at a given wavelength. The absorption maxima correspond to one or more electrons transitioning to an excited state.
| + | Climate change effects on wildlife include increases and changes in disease and pathogens distribution, patterns, and outbreaks in wildlife<ref>Bradley, B. A., Wilcove, D. S., and Oppenheimer, M., 2010. Climate change increases risk of plant invasion in the Eastern United States. Biological Invasions, 12, pp.1855-1872. [https://doi.org/10.1007/s10530-009-9597-y doi: 10.1007/s10530-009-9597-y]</ref><ref>Cudmore, T. J., Björklund, N., Carroll, A. L., and Lindgren, B. S., 2010. Climate change and the range expansion of an aggressive bark beetle: evidence of higher beetle reproduction in naïve host tree populations. Journal of Applied Ecology, 47(5), pp. 1036-1043. [https://doi.org/10.1111/j.1365-2664.2010.01848.x doi: 10.1111/j.1365-2664.2010.01848.x] [//www.enviro.wiki/images/b/b3/Cudmore2010.pdf Article pdf]</ref><ref>Price, S. J., Leung, W. T., Owen, C. J., Puschendorf, R., Sergeant, C., Cunningham, A. A., Balloux, F., Garner, T. W., and Nichols, R. A., 2019. Effects of historic and projected climate change on the range and impacts of an emerging wildlife disease. Global Change Biology, 25(8), pp. 2648-2660. [https://doi.org/10.1111/gcb.14651 doi: 10.1111/gcb.14651]</ref>; changes in range distributions and shifts in latitudinal and elevational gradients; changes in phenology or the timing of life cycle events that may create phenological mismatches<ref>Renner, S. S., and Zohner, C. M., 2018. Climate Change and Phenological Mismatch in Trophic Interactions Among Plants, Insects, and Vertebrates. Annual Review of Ecology, Evolution, and Systematics, 49, pp. 165-182. [https://doi.org/10.1146/annurev-ecolsys-110617-062535 doi: 10.1146/annurev-ecolsys-110617-062535]</ref>; and extinction or population reduction<ref name=":2">Urban, M. C., 2015. Accelerating extinction risk from climate change. Science, 348(6234), pp. 571-573. [https://doi.org/ 10.1126/science.aaa4984 doi: 10.1126/science.aaa4984] [//www.enviro.wiki/images/1/15/Urban2015.pdf Article pdf]</ref>. The effects of climate change across a species’ range will most likely not be homogenous, meaning it can vary substantially, especially if a species’ range spans across different continents as exhibited by many migratory birds. Other changes in habitat include shifting vegetation (i.e., tree-lines are shifting to higher elevations), changes in nutrients in plants, earlier snowmelt and run-off, increase in invasive species, warming of streams and rivers, reduction or degradation of habitat (i.e., glacial melt), and an increase in large wildfires<ref>Barbero, R., Abatzoglou, J. T., Larkin, N. K., Kolden, C. A., and Stocks, B., 2015. Climate change presents increased potential for very large fires in the contiguous United States. International Journal of Wildland Fire, 24(7), pp. 892-899. [https://doi.org/10.1071/WF150830128 doi: 10.1071/WF150830128] [//www.enviro.wiki/images/0/08/Barbero2015.pdf Article pdf]</ref>and droughts<ref>Schlaepfer, D. R., Bradford, J. B., Lauenroth, W. K., Munson, S. M., Tietjen, B., Hall, S. A., Wilson, S. D., Duniway, M. C., Jia, G., Pyke, D. A., Lkhagva, A., and Jamiyansharav, K., 2017. Climate change reduces extent of temperate drylands and intensifies drought in deep soils. Nature Communications, 8, pp. 14196. [https://doi.org/10.1038/ncomms14196 doi: 10.1038/ncomms14196]</ref>. |
| − | For a chemical bond to break via direct photolysis, a molecule must absorb a photon with higher energy than the energy of the bond. The energy of photons in the UV-Vis range is similar to the bond energies of several single [[wikipedia:Covalent_bond|covalent bonds]] found in organic molecules<ref name=":1" />. Therefore, many of the bonds in TNT, DNAN, NTO, and NQ are susceptible to photolysis from sunlight exposure (Figure 4).
| |
| − | [[File: PhotolysisFig4.png | thumb |380x380px| Figure 4. Calculated bond energies (black) and corresponding photon wavelengths (blue, determined from Planck’s equation<ref name=":1" />) required to break the bonds in TNT, DNAN, NTO, and NQ (calculations by Dr. Diego Troya<ref>Dontsova, K., Taylor, S., Brusseau, M. L., Simunek, J., and Hunt, E., 2017. Influence of climate on dissolution and phototransformation of NTO and DNAN from insensitive munitions and their fate in soils. <u>In</u>: Poster, the SERDP-ESTCP symposium, Washington, D.C., November 28-30, 2017. The Strategic Environmental Research and Development Program (SERDP) and Environmental Security Technology Certification Program (ESTCP).</ref>). Energies for bonds in the aromatic and heterocyclic rings are not shown because they exceed the maximum energy of the photons in sunlight reaching the Earth’s surface (500 kJ/mol or 239 nm).|left]][[File: PhotolysisFig5.png | thumb | 650px | Figure 5. Example of a UV photoreactor (A) with slots for UV bulbs (B) and a rotating carousel to hold samples (C). Reproduced with permission from [https://rayonet.org/ The Southern New England Ultraviolet Company].]] | |
| − | ==Photolysis Products and Pathways==
| |
| − | The products of the direct photolysis of MCs have been studied in depth, both in photoreactors using UV bulbs emitting light at a given wavelength (Figure 5) or wavelength ranges (including simulated sunlight) and outdoors in natural sunlight. The photolysis of MCs can form mineral products as well as transformation products that may be more toxic than the original compounds.
| |
| − | | |
| − | ''TNT''
| |
| − | | |
| − | The formation of pink and red wastewater released by some ammunition plants led to investigations into the photolysis products of TNT starting in the 1970s. Laboratory experiments observed rapid phototransformation from sunlight in natural waters, some with half-lives less than an hour<ref name=":3">Mabey, W.R., Tse, D., Baraze, A., and Mill, T., 1983. Photolysis of nitroaromatics in aquatic systems. I. 2,4,6-trinitrotoluene. Chemosphere, 12(1), pp. 3-16. [https://doi.org/10.1016/0045-6535(83)90174-1 doi: 10.1016/0045-6535(83)90174-1]</ref>. Numerous photolysis products of TNT have been reported and include, but are not limited to, 2-amino-4,6-dinitrobenzoic acid, 2,4,6-trinitrobenzaldehyde, 4,6-dinitroanthranil, 2,4,6-trinitrobenzonitrile, 2,4,6-trinitrobenzoic acid, [[wikipedia:1,3,5-Trinitrobenzene|1,3,5-trinitrobenzene]] (TNB), 2,4,6-trinitrobenzyl alcohol, and an array of [[wikipedia:Azo_compound|azo]] and [[wikipedia:Azoxy_compounds|azoxy]] compounds<ref name=":2" /><ref>Burlinson, N.E., Kaplan, L.A., and Adams, C.E., 1983. Photochemistry of TNT: Investigation of the ‘pink water’ problem. Naval Ordnance Laboratory, pp. 73-172. [https://apps.dtic.mil/sti/citations/AD0769670 AD076967] [[Special:FilePath/ADA1983.pdf| Report pdf]]</ref><ref name=":18">Spanggord, R.J., Mill, T., Chou, T., Mabey, W.R., Smith, J.H., and Lee, S., 1980. Environmental fate studies on certain munition wastewater constituents. Phase II - Laboratory studies. U S Army Biomedical Research and Development Laboratory. [https://apps.dtic.mil/sti/citations/ADA099256 ADA099256] [[Special:FilePath/ADA1980.pdf| Report pdf]]</ref><ref name=":4">Spanggord, R.J., Mabey, W.R., Mill, T., Tsong-Wen, C., Smith, J.H., Lee, S., and Roberts, D., 1983. Environmental fate studies on certain munitions wastewater constituents: Phase IV - Lagoon model studies. U S Army Biomedical Research and Development Laboratory. [https://apps.dtic.mil/sti/citations/ADA138550 ADA138550] [[Special:FilePath/ADA1983.pdf| Report pdf]]</ref><ref name=":5">Luning Prak D.J., Breuer J.E.T., Rios E.A., Jedlicka E.E., and O'Sullivan D.W., 2017. Photolysis of 2,4,6-trinitrotoluene in seawater and estuary water: Impact of pH, temperature, salinity, and dissolved organic matter. Marine Pollution Bulletin, 114(2), pp. 977-986. [https://doi.org/10.1016/j.marpolbul.2016.10.073 doi: 10.1016/j.marpolbul.2016.10.073]</ref>.
| |
| − | | |
| − | The mechanism of TNT photolysis is not completely understood as numerous products are formed, many of which are not readily synthesized or purchased<ref name=":5" />. However, evidence suggests that TNT is initially excited to a triplet state by UV light<ref name=":3" />. Figure 6 shows a proposed environmental reaction pathway for TNT that combines photo- and biological reactions based on studies in waste disposal lagoons<ref name=":4" />.
| |
| − | The aquatic toxicity of phototransformed TNT solutions and ammunition wastewaters was not found to be significantly different from that of untransformed TNT<ref>Liu, D.H.W., Spanggord, R.J., Bailey, H.C., Javitz, H.S., and Jones, D.C.L., 1983. Toxicity of TNT Wastewaters to Aquatic Organisms. Volume 1. Acute Toxicity of LAP Wastewater and 2,4,6-Trinitrotoluene. U S Army Medical Bioengineering Research and Development Laboratory. [https://apps.dtic.mil/sti/citations/ADA142144 ADA142144] [[Special:FilePath/ADA1983-2.pdf| Report pdf]]</ref><ref name=":13">Kennedy, A.J., Poda, A.R., Melby, N.L., Moores, L.C., Jordan, S.M., Gust, K.A., and Bednar, A.J., 2017. Aquatic toxicity of photo-degraded insensitive munition 101 (IMX-101) constituents. Environmental Toxicology and Chemistry, 36(8), pp.2050-2057. [https://doi.org/10.1002/etc.3732 doi: 10.1002/etc.3732]</ref>. Although TNT phototransformation products tend to be more toxic than TNT, the relatively low product formation coupled with the disappearance of TNT may explain these results.
| |
| − | | |
| − | ''RDX''
| |
| − | | |
| − | RDX photolysis products detected in aqueous systems include [[wikipedia:Nitrite|nitrite]], [[wikipedia:Nitrate|nitrate]], [[wikipedia:Ammoni|ammonia]], [[wikipedia:Formaldehyde|formaldehyde]], [[wikipedia:Formic_acid|formic acid]], [[wikipedia:Formamide|formamide]], [[wikipedia:Nitrous_oxide|nitrous oxide]], and 4-nitro-2,4-diazabutanal<ref>Just, C.L., and Schnoor, J.L., 2004. Phytophotolysis of Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) in Leaves of Reed Canary Grass. Environmental Science and Technology, 38(1), pp. 290-295. [https://doi.org/10.1021/es034744z doi: 10.1021/es034744z]</ref><ref name=":8">Bordeleau, G., Martel, R., Ampleman, G., and Thiboutot, S., 2013. Photolysis of RDX and nitroglycerin in the context of military training ranges. Chemosphere, 93(1), pp. 14-19. [https://doi.org/10.1016/j.chemosphere.2013.04.048 doi: 10.1016/j.chemosphere.2013.04.048]</ref><ref>Glover, D.J., and Hoffsommer, J.C., 1979. Photolysis of RDX in Aqueous Solution, With and Without Ozone. Naval Service Weapons Center, NSWC/WOL [https://apps.dtic.mil/sti/citations/ADA080195 TR 78-175]. [[Special:FilePath/ADA1979.pdf| Report pdf]]</ref><ref name=":7">Hawari, J., Halasz, A., Groom, C., Deschamps S., Paquet, L., Beaulieu, C., and Corriveau, A., 2002. Photodegradation of RDX in Aqueous Aolution: A Mechanistic Probe for Biodegradation with Rhodococcus sp. Environmental Science and Technology, 36(23), pp. 5117-5123. [https://doi.org/10.1021/es0207753 doi: 10.1021/es020775]</ref>. Stable phototransformation products also detected include nitroso compounds MNX (hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine), DNX (hexahydro-1,3-dinitroso-5-nitro-1,3,5-triazine), and TNX (hexahydro-1,3,5-trinitroso-1,3,5-triazine), which contain one, two, and three nitroso groups each, respectively, in place of the nitro groups. The formation of unsaturated MUX (1,3-dinitro-1,2,3,4-tetrahydro-1,3,5-triazine) was also reported (Figure 7)<ref name=":6" />.
| |
| − | | |
| − | Peyton et al. (1999) compiled experimental results for RDX photolysis in a proposed reaction mechanism (Figure 7)<ref name=":6" />. The most common first step is the breaking of an N-N bond between a [[wikipedia:Nitro_compound|nitro group]] and a nitrogen atom in the [[wikipedia:Heterocyclic_compound|heterocyclic ring]], resulting in two radical species (structure (R) in the center and an NO<sub>2</sub> radical). They hypothesize that the NO<sub>2</sub> radical can become an NO radical that may then recombine with structure (R), giving [[wikipedia:Nitroso|nitroso]] product MNX. This process can be repeated to form products DNX and TNX (i.e., via cleavage of the other N-N bonds, as shown in the formation of structure [R<sub>2</sub>]). The formation of MUX, containing an unsaturation (C=N double bond in the ring) was likely due to the loss of HNO<sub>2</sub> from RDX. Other products could include a combination of nitroso groups and unsaturations. The unsaturated compounds, including MUX, are expected to undergo [[wikipedia:Hydrolysis|hydrolysis]], forming many smaller products (e.g., nitrite and formaldehyde)<ref name=":6" /><ref name=":7" />. One of the major products of RDX photolysis is nitrate, a common groundwater pollutant of concern, despite being much less toxic than RDX<ref name=":8" />. The risk posed by the other toxic products should be evaluated, though they are formed in smaller quantities.
| |
| − | [[File: PhotolysisFig6.png | thumb |440x440px| Figure 6. Proposed environmental transformation pathway for TNT, including phototransformation and biotransformation reactions. Image courtesy of the U.S. Army Engineer Research and Development Center<ref name=":4" /><ref>Walsh, M.E., 1990. Environmental Transformation Products of Nitroaromatics and Nitramines: Literature Review and Recommendations for Analytical Method Development. US Army Corps of Engineers Cold Regions Research and Engineering Laboratory. [https://apps.dtic.mil/sti/citations/ADA220610 SR-90-2] [[Special:FilePath/ADA1990.pdf| Report pdf]]</ref>.|left]][[File: PhotolysisFig7.png | thumb | 650px | right | Figure 7. Proposed phototransformation mechanism for RDX. Image courtesy of the U.S. Army Engineer Research and Development Center<ref name=":6" />.]]
| |
| − | | |
| − | | |
| − | | |
| − | | |
| − | ''DNAN''
| |
| − | | |
| − | The products formed from DNAN photolysis include nitrate, nitrite, [[wikipedia:2,4-Dinitrophenol|2,4-dinitrophenol]] (DNP), 2-methoxy-5-nitrophenol, 4-methoxy-3-nitrophenol, [[wikipedia:Ammonium|ammonium]], formaldehyde, and formic acid<ref name=":9" /><ref>
| |
| − | Taylor, S., Walsh, M.E., Becher, J.B., Ringelberg, D.B., Mannes, P.Z., and Gribble, G.W., 2016. Photo-degradation of 2,4-dinitroanisole (DNAN): An emerging munitions compound. Chemosphere, 167, pp.193-203. [https://doi.org/10.1016/j.chemosphere.2016.09.142 doi:10.1016/j.chemosphere.2016.09.142]</ref><ref name=":10">Hawari, J., Monteil-Rivera, F., Perreault, N., Halasz, A., Paquet, L., Radovic-Hrapovic, Z., Deschamps, S., Thiboutot, S., Ampleman, G., 2015. Environmental fate of 2, 4-dinitroanisole (DNAN) and its reduced products. Chemosphere, 119, pp.16-23. [https://doi.org/10.1016/j.chemosphere.2014.05.047 doi: 10.1016/j.chemosphere.2014.05.047]</ref>.Rao et al. (2013) proposed a pathway for DNAN photolysis with photooxidation as the primary mechanism (Figure 8)<ref name=":9" />. According to the computational modeling of DNAN bond energies, the C-N bonds and the C-O bond of the [[wikipedia:Methoxy_group|methoxy group]] are most susceptible to photolysis (Figure 4)<ref name=":11">Qin, C., Abrell, L., Troya, D., Hunt, E., Taylor, S., and Dontsova, K., 2021. Outdoor dissolution and photodegradation of insensitive munitions formulations IMX-101 and IMX-104: Photolytic transformation pathway and mechanism study. Chemosphere, 280, 130672. [https://doi.org/10.1016/j.chemosphere.2021.130672 doi: 10.1016/j.chemosphere.2021.130672]</ref>. This is in agreement with the transformation products formed. Once DNAN is excited to a photo-activated triplet state, OH<sup>-</sup> in solution can displace either of its nitro groups via an [[wikipedia:SN2_reaction|S<sub>N</sub>2 reaction]] to form a methoxynitrophenol. This releases nitrite, which can then form nitrate through further photooxidation. DNP could form via ''O''-demethylation of DNAN, which could occur by [[wikipedia:Hydroxylation|hydroxylation]] of the [[wikipedia:Methyl_group|methyl group]] and the release of formaldehyde<ref>Studziński. W., Gackowska, A., Przybyłek, M., Gaca, J., 2017. Studies on the formation of formaldehyde during 2-ethylhexyl 4-(dimethylamino)benzoate demethylation in the presence of reactive oxygen and chlorine species. Environmental Science and Pollution Research, 24(9), pp. 8049-8061. [https://doi.org/10.1007/s11356-017-8477-8 doi:10.1007/s11356-017-8477-8] [[Special:FilePath/Studzinski2017.pdf| Article pdf]]</ref>. Formaldehyde or formic acid may react with NH<sub>2</sub> groups in methoxynitroanilines or aminonitrophenols to produce formamide derivatives<ref name=":10" />.
| |
| − | [[File: PhotolysisFig8.png | thumb |450x450px| left | Figure 8. Proposed phototransformation pathways for DNAN. Redrawn from Rao et al. (2013)<ref name=":9" />.]]
| |
| − | | |
| − | Ecotoxicity estimates predict that DNAN phototransformation products are less toxic than DNAN, except for DNP, which is more toxic and known to uncouple oxidative phosphorylation<ref name=":9" /><ref name=":11" />. However, upon further photolysis, DNP was shown to degrade into nitrocatechol and nitrite<ref name=":9" /><ref name=":10" />.
| |
| − | | |
| − | <br />''NTO''
| |
| − | | |
| − | Aqueous NTO formed ammonium, nitrate, nitrite, and a urazole intermediate when irradiated with UV light<ref name=":12" /><ref name=":14">Moores, L.C., Kennedy, A.J., May, L., Jordan, S.M., Bednar A. J., Jones S.J., Henderson D.L., Gurtowski L., and Gust, K.A., 2020. Identifying degradation products responsible for increased toxicity of UV-degraded insensitive munitions. Chemosphere, 240, 124958. [https://doi.org/10.1016/j.chemosphere.2019.124958. doi: 10.1016/j.chemosphere.2019.124958]</ref>. A significant amount of the original NTO mass was not recovered. This may be due, in part, to the volatilization of gaseous species since bubbles formed in the irradiated solutions. A proposed pathway for NTO photolysis is shown in Figure 9<ref name=":12" />. The first step is the breaking of the C-NO<sub>2</sub> bond, which has the lowest bond energy (Figure 4). This forms a nitro radical and a triazolone radical. The nitro radical can then form nitrite and nitrate, and the triazolone radical can form urazole upon reaction with water. Urazole and/or NTO may undergo hydrolysis that breaks open the heterocyclic ring and ultimately forms degradation products such as ammonium and carbon dioxide gas. In aquatic toxicity studies, UV-irradiated NTO was found to be up to 100 times more toxic than non-irradiated NTO<ref name=":13" /><ref name=":14" />. The specific photolysis products responsible for this increase in toxicity are still being investigated.
| |
| − | | |
| − | ''NQ''
| |
| − | | |
| − | The major products formed from NQ photolysis are nitrate, nitrite, [[wikipedia:Guanidine|guanidine]], and [[wikipedia:Urea|urea]]. Minor products include [[wikipedia:Cyanamide|cyanamide]], [[wikipedia:2-Cyanoguanidine|cyanoguanidine]], ammonium, [[wikipedia:Melamine|melamine]], [[wikipedia:Ammeline|ammeline]], and [[wikipedia:Cyanide|cyanide]]<ref name=":12" /><ref name=":14" /><ref name=":16">Burrows, W.D., Schmidt, M.O., Chyrek, R.H., and Noss, C.I., 1988. Photochemistry of aqueous nitroguanidine. US Army Biomedical Research and Development Laboratory. [https://apps.dtic.mil/sti/citations/ADA203200 ADA203200] [[Special:FilePath/ADA1988.pdf|Report pdf]]</ref><ref name=":19">Haag, W.R., Spanggord, R., Mill, T., Podoll, R.T., Chou, T., Tse, D.S., and Harper J.C., 1990. Aquatic environmental fate of nitroguanidine. Environmental Toxicology and Chemistry, 9(11), pp. 1359-1367. [https://doi.org/10.1002/etc.5620091105 doi: 10.1002/etc.5620091105]</ref><ref>Noss, C.I., and Chyreck, R.H., 1984. Nitroguanidine Wastewater Pollution Control Technology: Phase III. Treatment with Ultraviolet Radiation, Ozone, And Hydrogen Peroxide. U S Army Medical Bioengineering Research and Development Laboratory. [https://apps.dtic.mil/sti/citations/ADA139389 ADA13938] [[Special:FilePath/ADA1984.pdf|Report pdf]]</ref>. [[wikipedia:Methylnitronitrosoguanidine|Nitrosoguanidine]] and hydroxyguanidine were identified as intermediates that were further phototransformed<sup>30,31</sup>. A proposed pathway for NQ photolysis is shown in Figure 10<ref name=":12" />. The weakest bond is the N-NO<sub>2</sub> bond (Figure 4), and when it is cleaved, it forms nitro and guanidine radicals. The nitro radical can form nitrite and nitrate. The guanidine radical, upon reaction with a hydrogen ion or a water molecule, can form guanidine or hydroxyguanidine, respectively. Ammonia and cyanamide can also form from the breakdown of the guanidine radical. Cyanamide can then undergo either [[wikipedia:Dimer_(chemistry)|dimerization]] to form cyanoguanidine or hydrolysis to form urea. Melamine is a trimer of cyanamide and ammeline is a hydrolysis product of melamine. Cyanide may be formed from cyanoguanidine under acidic conditions<ref name=":13" />.
| |
| − |
| |
| − | UV-irradiated NQ was found to be orders of magnitude more toxic to aquatic organisms than NQ that was not irradiated<ref name=":13" /><ref name=":14" /><ref name=":23">
| |
| − | Gust, K.A., Stanley, J.K., Wilbanks, M.S., Mayo, M.L., Chappell P., Jordan, S.M., Moores, L.C., Kennedy, A.J., and Barker, N.D., 2017.. The increased toxicity of UV-degraded nitroguanidine and IMX-101 to zebrafish larvae: Evidence implicating oxidative stress. Aquatic Toxicology, 190, pp. 228-245. [https://doi.org/10.1016/j.aquatox.2017.07.004 doi: 10.1016/j.aquatox.2017.07.004]</ref><ref>van der Schalie, W.H.,1985. The toxicity of nitroguanidine and photolyzed nitroguanidine to freshwater aquatic organisms. U S Army Medical Bioengineering Research and Development Laboratory. [https://apps.dtic.mil/sti/citations/ADA153045 ADA153045] [[Special:FilePath/ADA1985.pdf|Report pdf]]</ref>. Furthermore, it was responsible for most of the toxicity of irradiated IMX-101. Moores et al. (2020) found that guanidine, nitrite, ammonia, nitrosoguanidine, and cyanide produced from NQ photolysis were each more toxic to ''[[wikipedia:Daphnia_pulex|Daphnia pulex]]'' than NQ, with nitrite and cyanide contributing the most to the toxicity<ref name=":14" />. When adding up the individual toxicities caused by these photolysis products, only 25% of the overall toxicity caused by exposure to irradiated NQ was accounted for. This implied that additional, unidentified products with greater toxicity to ''D. pulex'' formed and/or that exposure to all the products at once created a synergistic toxic effect.
| |
| − | [[File: PhotolysisFig9.png | thumb | 650px | Figure 9. Proposed phototransformation pathways for NTO. Redrawn from Becher et al. (2019)<ref name=":12" />.]]
| |
| − | [[File: PhotolysisFig10.png | thumb |470x470px| left | Figure 10. Proposed phototransformation pathways for NQ. Redrawn from Becher et al. (2019)<ref name=":12" />.]]
| |
| − | ==Photolysis Kinetics==
| |
| − | In dilute solutions, the direct photolysis of a compound may be described as a pseudo first-order process, in which the log of the compound concentration decreases linearly with time. In contrast, when the compound concentration is high enough that it absorbs most of the incident light, the photolysis rate no longer depends on the compound concentration, and the process may be described as zero order<ref name=":1" /><ref name=":17">Logan, S.R., 1997. Does a photochemical reaction have a reaction order? Journal of Chemical Education, 74(11), pp.1303. [https://doi.org/10.1021/ed074p1303 doi: 10.1021/ed074p1303]</ref>. First-order kinetics were reported in studies irradiating MCs at 1 mg L<sup>-1</sup>, whereas zero-order kinetics were reported for MCs irradiated at higher initial concentrations<ref name=":9" /><ref name=":15" /><ref name=":3" /><ref name=":16" /><ref name=":20">Moores, L.C., Jones, S.J., George, G.W., Henderson, D.L., and Schutt, T.C., 2020. Photo degradation kinetics of insensitive munitions constituents nitroguanidine, nitrotriazolone, and dinitroanisole in natural waters. Journal of Photochemistry and Photobiology A: Chemistry, 386, 112094. [https://doi.org/10.1016/j.jphotochem.2019.112094 doi: 10.1016/j.jphotochem.2019.112094]</ref>. The phase of an MC can impact its photolysis rate, as aqueous RDX was found to transform significantly faster than solid RDX<ref name=":8" />. In addition, the half-lives of UV-irradiated compounds are highly dependent on sunlight exposure and intensity, which vary with time (of day and year) and location (latitude and altitude). Therefore, it is more useful to describe photolysis in terms of quantum yields rather than reaction rates or half-lives, since they account for differences in experimental setup and irradiance and are more readily comparable<ref name=":17" />.
| |
| − | | |
| − | ==Quantum Yields==
| |
| − | The proportion of photons absorbed by a given compound that results in its transformation (i.e., via chemical reaction) is known as the reaction’s quantum yield. These are usually determined experimentally. Schwarzenbach et al. (2003)<ref name=":1" /> define quantum yield as:
| |
| − | [[File:PhotolysisEqn1.png|center|800px]]
| |
| − | In systems involving organic pollutants in natural waters, quantum yields are generally between 0 and 1, since chain reactions resulting in higher quantum yields are rare. Furthermore, quantum yields are not expected to vary significantly with the wavelength of light absorbed, at least over a given absorption band, and can be used to approximate the overall transformation rates of compounds<ref name=":1" />.Where quantum yield can be assumed to be independent of wavelength, it can be expressed as the ratio of the photolysis reaction rate (k<sub>p</sub>) to the rate of light absorption (k<sub>a</sub>):
| |
| − | [[File:PhotolysisEqn2.png|center|100px]]
| |
| − | Thus, if there is an experimentally derived quantum yield available, and if the light absorption rate of a given system can be measured or approximated (e.g., by a spreadsheet or computer program), the direct photolysis rate can be calculated by the above equation. Reported quantum yields for solutions in distilled water are included in the table below:
| |
| − | {| class="wikitable" style="margin: auto; text-align: center;"
| |
| − | |'''Munitions Compounds '''
| |
| − | |'''Light source'''
| |
| − | |'''Reaction quantum yield'''
| |
| − | |-
| |
| − | |TNT
| |
| − | |313 and 366 nm
| |
| − | |2.7 x 10<sup>-3</sup> <ref name=":3" />
| |
| − | |-
| |
| − | |TNT
| |
| − | |Simulated sunlight
| |
| − | |2.6 x 10<sup>-3</sup> <ref name=":5" />
| |
| − | |-
| |
| − | |RDX
| |
| − | |313 nm
| |
| − | |1.6 x 10<sup>-1</sup> <ref name=":18" />
| |
| − | |-
| |
| − | |DNAN
| |
| − | |312 nm max (280-315 nm)
| |
| − | |3.7 x 10<sup>-4</sup> <ref name=":9" />
| |
| − | |-
| |
| − | |DNAN
| |
| − | |350 nm max (316-400 nm)
| |
| − | |2.9 x 10<sup>-4</sup> <ref name=":9" />
| |
| − | |-
| |
| − | |DNAN
| |
| − | |Simulated sunlight
| |
| − | |1.1 x 10<sup>-4</sup> <ref name=":9" />
| |
| − | |-
| |
| − | |DNAN
| |
| − | |Sunlight
| |
| − | |3.2 x 10<sup>-3</sup> <ref name=":20" />
| |
| − | |-
| |
| − | |NTO
| |
| − | |Sunlight
| |
| − | |5.9 x 10<sup>-5</sup> <ref name=":20" />
| |
| − | |-
| |
| − | |NQ
| |
| − | |Sunlight
| |
| − | |1.0 x 10<sup>-2</sup> <ref name=":20" />
| |
| − | |-
| |
| − | |NQ
| |
| − | |Sunlight
| |
| − | |1.02 x 10<sup>-2</sup>, 1.26 x 10<sup>-2</sup> <ref name=":19" />
| |
| − | |}
| |
| − | The quantum yield of a compound can be determined experimentally by measuring the direct photolysis rate in a controlled system and measuring the rate of light absorption. The latter is calculated by measuring the irradiance via chemical or physical methods (e.g., by irradiating a chemical with a known quantum yield and readily quantifiable reaction products) combined with absorption data from UV-Vis spectra<ref name=":1" /><ref name=":9" /><ref name=":20" />.
| |
| − | | |
| − | ==Indirect Photolysis==
| |
| − | When some compounds absorb photons that promote their electrons to an excited state, they can transfer this energy to nearby molecules instead of being chemically transformed themselves. These compounds are called photosensitizers. MCs can undergo transformation via indirect photolysis in the presence of photosensitizers such as natural organic matter, which contains light-absorbing [[wikipedia:Chromophore|chromophores]] and is found in soil and surface water<ref>MacCarthy, P., and Suffet, I.H., 1988. PREFACE, Introduction to Aquatic Humic Substances and Their Influence on the Fate and Treatment of Pollutants. <u>In</u>: I.H. Suffet and P. MacCarthy (eds.). Aquatic Humic Substances and Their Influence on the Fate and Treatment of Pollutants. American Chemical Society, Washington, D.C., pp. xiii -xxx. ISBN: 9780841214286/eISBN: 9780841224018 [https://pubs.acs.org/doi/abs/10.1021/ba-1988-0219.pr001 doi: 10.1021/ba-1988-0219.pr001] [[Special:FilePath/MacCarthy1988.pdf| Chapter pdf]]</ref><ref>Schwarzenbach, R.P., Gschwend. P.M., and Imboden, D.M.,2003. Chapter 16 - Indirect Photolysis: Reactions with Photooxidants in Natural Waters and in the Atmosphere. <u>In</u>: R.P. Schwarzenbach, P.M. Gschwend, and D.M. Imboden (eds.) Environmental organic chemistry. 2nd ed. John Wiley & Sons, Inc., Hoboken, NJ, pp. 655-686. ISBN: 9780471350538/eISBN: 9780471649649 [https://doi.org/10.1002/0471649643 doi:10.1002/0471649643.ch16]</ref>. This transformation occurs if the photosensitizer reacts directly with the MC or if it forms reactive species, such as [[wikipedia:Hydroxyl_radical|hydroxyl radicals]] and singlet oxygen, that then (in most cases) oxidize the MC. Nitrate, nitrite, and iron-containing complexes are also important photosensitizers in natural waters that generate reactive species<ref>Blough, N.V., and Zepp, R.G., 1995. Reactive Oxygen Species in Natural Waters. In: C.S. Foote, J.S. Valentine, A. Greenberg, J.F. Liebman (eds.) Active Oxygen in Chemistry. Structure Energetics and Reactivity in Chemistry Series (SEARCH Series), Vol 2. Springer, Dordrecht, Netherlands, pp. 280-333. ISBN: 9780751403718/eISBN: 9789400708747 [https://doi.org/10.1007/978-94-007-0874-7_8 doi: 10.1007/978-94-007-0874-7_8]</ref>.
| |
| − | | |
| − | Environmentally relevant concentrations of [[wikipedia:Humic_substance|humic]] and [[wikipedia:Fulvic_acid|fulvic]] acids either inhibited or had no effect on insensitive MC photolysis rates<ref name=":9" /><ref name=":20" />. However, humic substances were suspected of acting as triplet sensitizers of TNT upon UV irradiation and increased phototransformation rates<ref name=":3" /><ref name=":5" />. Several studies demonstrated that the photolysis of MCs was enhanced in the presence of TiO<sub>2</sub> nanoparticles and [[wikipedia:Fenton's_reagent|Fenton’s reagent]], likely due to the production of hydroxyl radicals<ref name=":9" /><ref>Le Campion, L., Giannotti, C., Ouazzani, J., 1999. Photocatalytic degradation of 5-nitro-1,2,4-triazol-3-one NTO in aqueous suspention of TiO2. Comparison with fenton oxidation. Chemosphere, 38(7), pp. 1561-1570. [https://doi.org/10.1016/S0045-6535(98)00376-2 doi: 10.1016/S0045-6535(98)00376-2]</ref><ref name=":22">Halasz, A., Hawari, J., and Perreault, N.N., 2018. New Insights into the Photochemical Degradation of the Insensitive Munition Formulation IMX-101 in Water. Environmental Science and Technology, 52(2), pp. 589-596. [https://doi.org/10.1021/acs.est.7b04878 doi: 10.1021/acs.est.7b04878</ref><ref>Liu, Z., He, Y., Li, F., Liu, Y., 2006. Photocatalytic Treatment of RDX Wastewater with Nano-Sized Titanium Dioxide (5 pp). Environmental Science and Pollution Research, 13(5), pp. 328-332. [https://doi.org/10.1065/espr2006.08.328 doi: 10.1065/espr2006.08.328]</ref><ref>Schmelling, D.C., and Gray, K.A., 1995. Photocatalytic transformation and mineralization of 2,4,6-trinitrotoluene (TNT) in TiO2 slurries. Water Research, 29(12), pp. 2651-2662. [https://doi.org/10.1016/0043-1354(95)00136-9 doi: 10.1016/0043-1354(95)00136-9]</ref>.
| |
| − | | |
| − | Co-contaminants can hinder the photolysis of a compound of interest by absorbing some of the incident light. They can also serve as photosensitizers that further transform the compound, in some cases producing additional products. Since MCs are usually released into the environment in formulations containing TNT, RDX, DNAN, NTO, and/or NQ, it is important to determine if there are any photochemical interactions between these compounds. Halasz et al. (2018) reported that DNAN, NTO, and NQ in irradiated IMX-101 all phototransformed at slower rates than the individual irradiated compounds<ref name=":22" /> In addition, methoxydinitrophenols formed when IMX-101 and IMX-104 were irradiated, which were not detected when DNAN was irradiated alone<ref name=":11" /><ref name=":22" />. This may have occurred by the simultaneous photonitration of methoxynitrophenols (formed from DNAN photolysis) and photodenitration of NQ and NTO.
| |
| | | | |
| − | ==Outdoor Phototransformation==
| + | Although climate change effects on wildlife often are linked to species-specific traits, there are general impacts associated with taxonomic groups<ref name=":1" />. For fish it can affect reproduction<ref name=":4">Pankhurst, N. W., and Munday, P. L., 2011. Effects of climate change on fish reproduction and early life history stages. Marine and Freshwater Research, 62(9), pp. 1015-1026. [https://doi.org/10.1071/MF10269 doi: 10.1071/MF10269] [[Special:FilePath/Pankhurst2011.pdf| Article pdf]]</ref>, growth, and recruitment<ref name=":3">Lynch, A. J., Myers, B. J. E., Chu, C., Eby, L. A., Falke, J. A., Kovach, R. P., Krabbenhoft, T. J., Kwak, T. J., Lyons, J., Paukert, C. P., and Whitney, J. E., 2016. Climate Change Effects on North American Inland Fish Populations and Assemblages. Fisheries, 41(7), pp. 346-361. [https://doi.org/10.1080/03632415.2016.1186016 doi: 10.1080/03632415.2016.1186016]</ref>(Figure 2). Cold-water fish such as inland North American species are highly affected with the warming of streams and rivers<ref name=":3" />. Amphibians are highly sensitive to their environment and changes in temperature and moisture can affect development, range, abundance, and phenology<ref>Blaustein, A.R., Walls, S.C., Bancroft, B.A., Lawler, J.J., Searle, C.L., and Gervasi, S.S., 2010. Direct and Indirect Effects of Climate Change on Amphibian Populations. Diversity, 2(2), pp. 281-313.[https://doi.org/10.3390/d2020281 doi: 10.3390/d2020281] [[Media:Blaustein 2010.pdf | Article pdf]]</ref><ref name=":1" /><ref>Ficetola, G. F., and Maiorano, L., 2016. Contrasting effects of temperature and precipitation change on amphibian phenology, abundance and performance. Oecologia, 181(3), pp. 683-693. [https://doi.org/10.1007/s00442-016-3610-9 doi: 10.1007/s00442-016-3610-9]</ref>. In reptiles, climate change effects can alter thermoregulation patterns, affect female reproduction and in some species, change sex ratios with increasing temperature<ref name=":1" />. Furthermore, for many bird species the timing of migration and other phenological events are affected by climate change<ref>Crick, H. Q. P., 2004. The impact of climate change on birds. Ibis, 146(s1), pp. 48-56. [https://doi.org/10.1111/j.1474-919X.2004.00327.x doi: 10.1111/j.1474-919X.2004.00327.x] [//www.enviro.wiki/images/c/c9/Crick2004.pdf Article pdf]</ref>. Range shifts, growth size, and survival are linked to climate change for mammals<ref name=":1" />. Arctic marine mammals are closely linked to sea ice dynamics and a changing climate will affect these dynamics<ref>Kovacs, K. M., Lydersen, C., Overland, J. E., and Moore, S. E., 2011. Impacts of changing sea-ice conditions on Arctic marine mammals. Marine Biodiversity, 41, pp. 81-194. [https://doi.org/10.1007/s12526-010-0061-0 doi: 10.1007/s12526-010-0061-0]</ref>. Therefore, it is increasingly important for conservation and management plans to consider the effects of climate change on wildlife and habitat for the geographic location<ref>Mawdsley, J. R., O’Malley, R., and Ojima, D. S. 2009. A Review of Climate-Change Adaptations Strategies For Wildlife Management and Biodiversity Conservation. Conservation Biology, 23(5), pp. 1080-1089. [https://doi.org/10.1111/j.1523-1739.2009.01264.x doi: 10.1111/j.1523-1739.2009.01264.x]</ref>. |
| − | [[File: PhotolysisFig11.png | thumb |550x550px| left | Figure 11. Comparison of solid mass loss and recovered dissolved mass of particles of TNT, Tritonal, Comp B, and C4 exposed to sunlight and precipitation over a period of three years. Each point represents a separate particle. Over 60% of the solid mass lost, as measured by electronic balance, was not recovered as dissolved compounds analyzed with the HPLC. These unaccounted mass losses scaled closely with the surface area of each particle and increased with time. Plotted with data from Taylor et al. (2010)<ref name=":24" />.]] [[File: PhotolysisFig12.png | thumb | 650px | Figure 12. Mass balances showing the fate of solid munitions particles deposited on the ground and exposed to precipitation and sunlight for three years (traditional munitions; average for TNT, Tritonal, Comp B, and C4) and two years (insensitive munitions; average for IMX-101, IMX-104, and PAX-21). Plotted with data from Taylor et al. (2010)43 and Taylor et al. (2015)<ref name=":25" />.]]Mass balance studies were conducted in which solid, centimeter-sized particles of munitions were exposed to sunlight and precipitation. The traditional munitions tested included TNT, Tritonal (contains TNT), Comp B (contains TNT and RDX), and C4 (contains RDX)<ref name=":24">Taylor, S., Lever, J., Walsh. M., Fadden, J., Perron, N., Bigl, S., Spanggord, R., Curnow, M., and Packer, B., 2015. Dissolution Rate, Weathering Mechanics, and Friability of TNT, Comp B, Tritonal, And Octol. U.S. Army Engineer Research and Development Center (ERDC)/ Cold Region Research and Engineering Laboratory (CRREL) [https://apps.dtic.mil/sti/citations/ADA518701 TR-10-2]. [[Special:FilePath/ERDC2015.pdf| Report pdf]]</ref>. The particles were placed in funnels attached to covered bottles to collect effluent precipitation and any dissolved species. At the end of the three-year experiment, the remaining particles were weighed to determine the total solid mass loss. This loss was compared with the recovery of dissolved mass collected in the bottles and analyzed using [[wikipedia:High-performance_liquid_chromatography|High Performance Liquid Chromatography]] (HPLC). Compounds analyzed included TNT, RDX, [[wikipedia:HMX|HMX]] (octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine, a byproduct of RDX production), and several TNT transformation products. More than half of the solid mass loss was not accounted for and was likely due to surface phototransformation to products not analyzed with HPLC and subsequent dissolution (Figure 11).
| |
| − | | |
| − | Similar experiments were conducted with insensitive munitions formulations IMX-101, IMX-104, and PAX-21 (contains RDX, DNAN, and [[wikipedia:Ammonium_perchlorate|ammonium perchlorate]]) over a period of two years<ref name=":25">Taylor, S., Dontsova, K., Walsh, M.E., and Walsh, M.R., 2015. Outdoor dissolution of detonation residues of three insensitive munitions (IM) formulations. Chemosphere, 134, pp. 250-256. [https://doi.org/10.1016/j.chemosphere.2015.04.041 doi: 10.1016/j.chemosphere.2015.04.041]</ref><ref>SERDP, 2018. Dissolution of NTO, DNAN, and Insensitive Munitions Formulations and Their Fates in Soils. Prepared by K. Dontsova, S. Taylor, and R. Pesce-Rodriguez, Project No: [https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminants-on-Ranges/Characterizing-Fate-and-Transport/ER-2220/ER-2220/(modified)/15Jan2016 ER-2220], Strategic Environmental Research and Development Program, Arlington, VA, February 2018.</ref>. Because insensitive MCs are more soluble in water than traditional MCs, they dissolved more readily with precipitation and received less sunlight exposure overall. Thus, the relative amount of solid mass loss that was not recovered as dissolved mass, as determined by HPLC, was smaller for the insensitive MCs than for the traditional MCs likely because of faster particle dissolution and less phototransformation to unidentified products (Figure 12).
| |
| − | ==Summary and Implications for Remediation==
| |
| − | Photolysis is an important pathway for MCs deposited on soils and in surface waters since they absorb light in the UV-Vis range. The rate of MC photolysis depends on the location (i.e., sunlight intensity) and the local environmental matrix (photosensitizers, co-contaminants). Experimentally determined quantum yields can provide an estimate of MC photolysis rates if the UV irradiance is known. Although photolysis can result in a decrease in MC concentrations, it produces compounds that may be more toxic. In particular, the irradiation of NQ, both alone and in IMX-101, generates products that are significantly more toxic to aquatic organisms than NQ. This has been attributed to the products having a higher potential of inducing oxidative stress<ref name=":23" />. Due to the order-of-magnitude differences in aqueous solubility between MCs, (least soluble) RDX < TNT < DNAN < NQ < NTO (most soluble)<ref>Taylor, S., Ringelberg, D.B., Dontsova, K., Daghlian, C.P., Walsh, M.E., and Walsh, M.R., 2013. Insights into the dissolution and the three-dimensional structure of insensitive munitions formulations. Chemosphere, 93(9), pp. 1782-1788. [https://doi.org/10.1016/j.chemosphere.2013.06.011 doi: 10.1016/j.chemosphere.2013.06.011]</ref>, it is likely that RDX, TNT, and DNAN from surface-deposited munitions will undergo photolysis primarily in the solid form and NQ and NTO will undergo photolysis in the aqueous form prior to percolating into the soil. Outdoor exposure studies suggest that a significant fraction of munitions deposited as particles onto soil surfaces is phototransformed. To maximize the sunlight exposure of future MC deposition, Bordeleau et al. (2013) suggest keeping range grasses short<ref name=":8" />.
| |
| | | | |
| | ==References== | | ==References== |
| | <references /> | | <references /> |
| | + | |
| | ==See Also== | | ==See Also== |