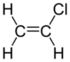Difference between revisions of "User:Admin/sandbox"
| Line 5: | Line 5: | ||
| − | <div class="toccolours mw-collapsible mw-collapsed" style="width: | + | <div class="toccolours mw-collapsible mw-collapsed" style="width:80%"> |
Table 2. This is what the table would look like in a collapsed box | Table 2. This is what the table would look like in a collapsed box | ||
<div class="mw-collapsible-content"> | <div class="mw-collapsible-content"> | ||
Revision as of 16:22, 28 October 2015
I have installed SandboxLink extension that provides each user their own sandbox accessible through their personal menu bar (top right)
CONTRIBUTOR(S):
INTRODUCTION
Chlorinated solvents are a large family of organic solvents that contain chlorine chlorine atoms in their molecular structure. They were first produced in Germany in the 1800s, and widespread use in the United States (U.S.) began after World War II. In the period of 1940-1980, the U.S. produced about 2 billion pounds of chlorinated solvents each year [1]. Chlorinated solvents, including carbon tetrachloride (CT), 1,1,1-trichloroethane (TCA), perchloroethene or tetrachloroethene (PCE) and trichloroethene (TCE) have been among the most widely used cleaning and degreasing solvents in the U.S [2]. They also have been used in a wide variety of other purposes such as adhesives, chemical intermediates, clothes, pharmaceuticals, pesticides, and textile processing.
PHYSICAL and CHEMICAL PROPERTIES
Chlorinated solvents are organic compounds generally constructed of a simple hydrocarbon chain (typically one to three carbon atoms in length). They can be divided into three categories based on their structural characteristics: chlorinated methanes, chlorinated ethanes and chlorinated ethenes.
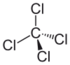
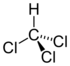
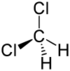
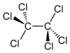
Chlorinated methanes represent the most structurally simple solvent class and consist of a single carbon center (known as a methyl carbon) to which as many as four chlorine atoms are bonded. From the perspective of groundwater contamination, perhaps the most well-known chlorinated methanes are carbon tetrachloride (CT) or tetrachloromethane, trichloromethane (commonly known as chloroform [CF]), dichloromethane (DCM), or methylene chloride (MC) and chloromethane (CM), or methyl chloride.
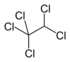
Chlorinated ethanes consist of two carbon centers joined by a single covalent bond. The most frequently encountered groundwater pollutants of this class include 1,1,1-trichloroethane (1,1,1-TCA) and 1,2-dichloroethane.
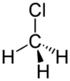
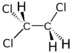
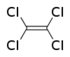
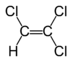
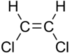
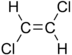
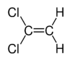
Chlorinated ethenes (also referred to as chlorinated ethylenes) also possess two carbon centers, but unlike chlorinated ethanes, these carbon atoms are joined by a carbon-carbon double bond. Chlorinated ethenes that are important groundwater contaminants include tetrachloroethene, or perchloroethene (PCE), trichloroethene (TCE), dichloroethene (DCE)) (DCE, mainly two geometric isomers cis-1,2-dichloroethene and trans-1,2-dichloroethene), and vinyl chloride (VC). Nomenclature and structure of selected compounds from each solvent class are shown in Table 1.
| IUPAC Name | Common Name | Abbreviation/Acronym | CAS Registry Number | Molecular Formula | Chemical Structure |
| Chlorinated Methanes | |||||
| tetrachloromethane | carbon tetrachloride | CT | 56-23-5 | CCl4 | |
| trichloromethane | chloroform | CF | 67-66-3 | CHCl3 | |
| dichloromethane | methylene chloride | DCM | 75-09-2 | CH2Cl2 | |
| chloromethane | methyl chloride | CM | 74-87-3 | CH3Cl | |
| Chlorinated Ethanes | |||||
| hexachloroethane | perchloroethane | HCA | 67-72-1 | C2Cl6 | |
| pentachloroethane | - | PCA | 76-01-7 | C2HCl5 | |
| 1,1,1,2-tetrachloroethane | - | 1,1,1,2-TeCA | 630-20-6 | C2H2Cl4 | |
| 1,1,2,2-tetrachloroethane | - | 1,1,2,2-TeCA | 79-34-5 | C2H2Cl4 | |
| 1,1,2-trichloroethane | - | 1,1,2-TCA | 79-00-5 | C2H3Cl3 | |
| 1,1,1-trichloroethane | methyl chloroform | 1,1,1-TCA | 71-55-6 | C2H3Cl3 | |
| 1,2-dichloroethane | - | 1,2-DCA | 107-06-2 | C2H4Cl2 | |
| 1,1-dichloroethane | - | 1,1-DCA | 75-34-3 | C2H4Cl2 | |
| chloroethane | - | CA | 75-00-3 | C2H5Cl | |
| Chlorinated Ethenes | |||||
| tetrachloroethene | perchloroethene | PCE | 127-18-4 | C2Cl4 | |
| trichloroethene | - | TCE | 79-01-6 | C2HCl3 | |
| cis-1,2-dichloroethene | cis-dichloroethene | cis-DCE | 156-59-2 | C2H2Cl2 | |
| trans-1,2-dichloroethene | trans-dichloroethene | trans-DCE | 156-60-5 | C2H2Cl2 | |
| 1,1-dichloroethene | vinylidene chloride | 1,1-DCE | 75-35-4 | C2H2Cl2 | |
| chloroethene | vinyl chloride | VC | 75-01-4 | C2H3Cl | |
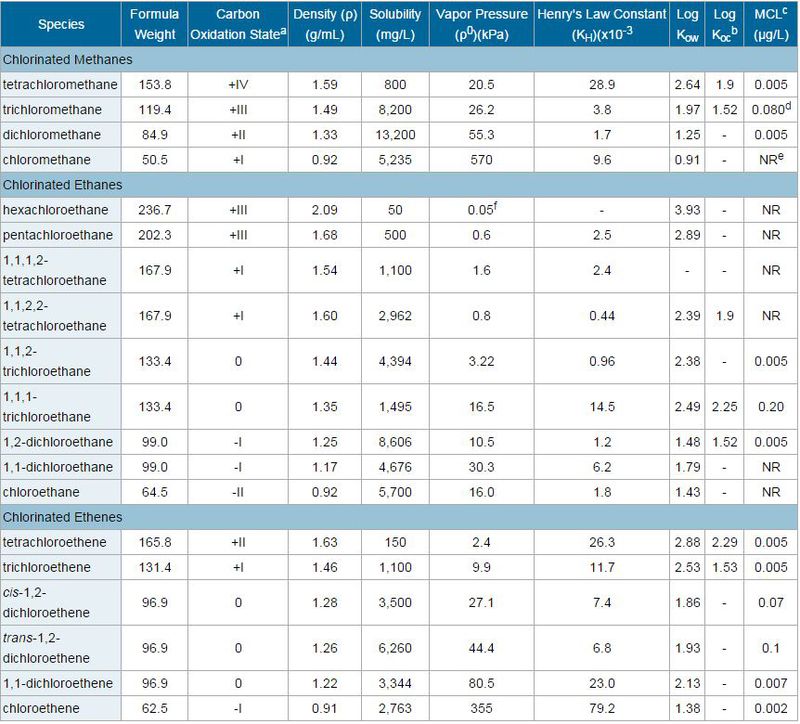
The chlorinated solvents and many of their transformation products are colorless liquids at room temperature. They are heavier than water with densities greater than 1 gram per cubic centimeter (g/cm3) which means they can penetrate deeply into an aquifer. Some physical and chemical properties of most widely used chlorinated solvents are listed in Table 2.
| Species | Formula Weight | Carbon Oxidation Statea | Density (ρ)(g/mL) | Solubility (mg/L) | Vapor Pressure (ρ0)(kPa) | Henry's Law Constant (KH)(x10-3 | Log Kow | Log Kocb | MCLc (μg/L) |
| Chlorinated Methanes | |||||||||
| tetrachloromethane | 153.8 | +IV | 1.59 | 800 | 20.5 | 28.9 | 2.64 | 1.9 | 0.005 |
| trichloromethane | 119.4 | +III | 1.49 | 8,200 | 26.2 | 3.8 | 1.97 | 1.52 | 0.080d |
| dichloromethane | 84.9 | +II | 1.33 | 13,200 | 55.3 | 1.7 | 1.25 | - | 0.005 |
| chloromethane | 50.5 | +I | 0.92 | 5,235 | 570 | 9.6 | 0.91 | - | NRe |
| Chlorinated Ethanes | |||||||||
| hexachloroethane | 236.7 | +III | 2.09 | 50 | 0.05f | - | 3.93 | - | NR |
| pentachloroethane | 202.3 | +III | 1.68 | 500 | 0.6 | 2.5 | 2.89 | - | NR |
| 1,1,1,2-tetrachloroethane | 167.9 | +I | 1.54 | 1,100 | 1.6 | 2.4 | - | - | NR |
| 1,1,2,2-tetrachloroethane | 167.9 | +I | 1.60 | 2,962 | 0.8 | 0.44 | 2.39 | 1.9 | NR |
| 1,1,2-trichloroethane | 133.4 | 0 | 1.44 | 4,394 | 3.22 | 0.96 | 2.38 | - | 0.005 |
| 1,1,1-trichloroethane | 133.4 | 0 | 1.35 | 1,495 | 16.5 | 14.5 | 2.49 | 2.25 | 0.20 |
| 1,2-dichloroethane | 99.0 | -I | 1.25 | 8,606 | 10.5 | 1.2 | 1.48 | 1.52 | 0.005 |
| 1,1-dichloroethane | 99.0 | -I | 1.17 | 4,676 | 30.3 | 6.2 | 1.79 | - | NR |
| chloroethane | 64.5 | -II | 0.92 | 5,700 | 16.0 | 1.8 | 1.43 | - | NR |
| Chlorinated Ethenes | |||||||||
| tetrachloroethene | 165.8 | +II | 1.63 | 150 | 2.4 | 26.3 | 2.88 | 2.29 | 0.005 |
| trichloroethene | 131.4 | +I | 1.46 | 1,100 | 9.9 | 11.7 | 2.53 | 1.53 | 0.005 |
| cis-1,2-dichloroethene | 96.9 | 0 | 1.28 | 3,500 | 27.1 | 7.4 | 1.86 | - | 0.07 |
| trans-1,2-dichloroethene | 96.9 | 0 | 1.26 | 6,260 | 44.4 | 6.8 | 1.93 | - | 0.1 |
| 1,1-dichloroethene | 96.9 | 0 | 1.22 | 3,344 | 80.5 | 23.0 | 2.13 | - | 0.007 |
| chloroethene | 62.5 | -I | 0.91 | 2,763 | 355 | 79.2 | 1.38 | - | 0.002 |
They are relatively volatile compounds with relatively high Henry’s Law constants(KH, a measure of the strength of partitioning from water into air). Generally, when KH for a compound exceeds 0.2 atmosphere/mole fraction (atm/M), they can readily be removed from water by air stripping it. Most chlorinated solvents can be classified as sparingly soluble in water, with aqueous solubilities generally on the order of 10s to 100s of mg/L (Table 2). As the number of chlorine atoms on a compound increases, the solubility decreases. Because of their relatively low solubilities, chlorinated solvents dissolve slowly in groundwater. Another consequence of their limited solubility is their tendency to occur in the subsurface as a separate immiscible liquid phase which, because of its density compared to water, tends to sink in groundwater. Under these conditions, these are referred to as dense non-aqueous phase liquid (DNAPL). Although chlorinated solvents are not very soluble in water, their solubility is typically orders of magnitude greater than their established drinking water standards.
Chlorinated solvents can be considered moderately hydrophobic which can be determined by their octanol-water partition coefficients (Kow, a measure of the tendency of a substance to prefer an organic or oily phase rather than an aqueous phase). Log Kow values less than 3 indicate that the compound does not sorb strongly to aquifer solids, but can be removed readily by activated carbon. On the other hand, compounds with log Kow less than 2, such as VC, generally are not removed well by activated carbon either. [3]
ENVIRONMENTAL CONCERN
For decades, widespread use and improper storage/disposal practices of these solvents has impacted underlying soils and groundwater, creating a significant environmental problem and human health risk. This problem became most evident following the passage in the United States of the Comprehensive Environmental Response, Compensation and Liability Act (CERCLA), or Superfund legislation, in 1980 and the subsequent evaluation of chemical contamination of groundwater. Over 5,000 Department of Defense (DoD), Department of Energy (DOE), and Superfund National Priorities List (NPL) sites are contaminated with chlorinated solvents [4] , with TCE being the most frequently detected contaminant (e.g., >60% of NPL sites).[5]. Additionally, there are approximately 36,000 active drycleaner sites across the United States; 75% of these facilities are suspected to have soil and/or groundwater contamination from solvent releases[6]. According to the USEPA Toxic Release Inventory, an average of 11 million pounds of TCE and 3 million pounds of PCE were released between 1998 and 2001[7].
The USEPA characterized TCE as “carcinogenic in humans by all routes of exposure” [8] and classified PCE as “likely to be carcinogenic to humans”.[9] Exposure to TCE and PCE has been linked to an increased risk of kidney cancer in humans [10] and has caused liver, kidney, lung, and testicular tumors in mice and rat studies. [11] Furthermore, TCE and PCE are ranked sixteenth and thirty-third, respectively, on the 2013 Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) Priority List of Hazardous Substances based on their toxicity, frequency of occurrence at NPL sites, and potential for human exposure.[10] The close proximity of chlorinated solvent sites (e.g., drycleaners, NPL, industrial) to urban areas has led to numerous impacts to public and private water supply wells;[12] between 9% and 34% of drinking water supply sources have some level of TCE contamination.[13]
Since most of the commonly used chlorinated solvents are classified as “known” or “potential” carcinogens; they are regulated with strict drinking water standards (also referred as maximum contaminant levels [MCLs]]). Many chlorinated solvents are considered to present health risks if ingested in drinking water at concentrations greater than 5 micrograms per liter (µg/L) (5 parts per billion [ppb]). Also, due to their relatively high volatility, vapors from groundwater plumes can also pose unacceptable risks at some sites. When the MCL is compared to concentrations of hundreds or thousands of ppb that are commonly observed in groundwater at chlorinated solvent sites, it becomes apparent that even a small release can lead to a significant environmental problem. Today, it is recognized that there are thousands of public and private sites with chlorinated solvent related groundwater contamination problems. The groundwater plumes we see today were largely caused by releases that occurred in the 1960s, 1970s, and 1980s, illustrating the persistent long-term aspects of the chlorinated solvent problem .[14]
Fate and Transport
The physical, chemical and biological properties of chlorinated solvents and the nature of the subsurface media through which the compounds are migrating affect their fate and transport in the environment and potential remediation strategies. Figure 1 illustrates a typical distribution of chlorinated solvents discharged into subsurface. The solvent migrates down through the unsaturated (vadose) zone, probably leaving some residual solvent behind as it follows the path of least resistance. Eventually, it may encounter groundwater that forms the aquifer of a potential groundwater supply. Since the chlorinated solvents are denser than water, the downward movement continues within the subsurface via gravity along a permeable pathway, potentially spreading laterally or changing directions as less permeable material is encountered. Soils and aquifer solids containing sand and gravel material that is relatively large in diameter and relatively porous, allows good passage of both water and chlorinated solvent liquids through them. [15]
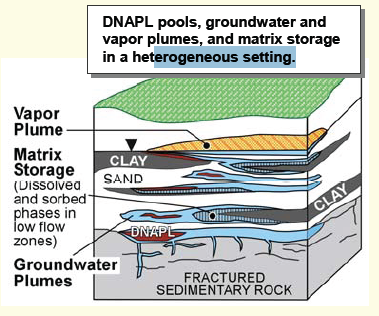
Other subsurface materials such as silts and clays are very fine and may be relatively impervious to water and solvents. Chlorinated solvents migrating vertically downward are subject to a variety of influences including capillary forces imposed by the various types of subsurface soils that are encountered, and organic carbon content in soils. When chlorinated solvents meet clay layers in their downward migration, they may pool on top of the clay, sorb into the clays, and/or seek a downward passage around the clay layer. Even when chlorinated solvents encounter more permeable sand and gravel layers, they may be diverted into one or the other because of these influences.
The liquid solvent (or DNAPL) present in soil, subsurface solids and groundwater represents the “source” of groundwater contamination. DNAPLs have very low aqueous solubilities that may exceed regulatory criteria by as much as five orders of magnitude [1]; as a result, these compounds only slowly dissolve in groundwater and act as long-term sources of groundwater contamination. Over time, constituents in DNAPL dissolve in water and/or volatilize into soil gas. This process leads to plume formation in transmissive zones where there is flow. At the same time, high concentrations of dissolved contaminants in transmissive zones drive contaminants into low permeability zones via diffusion. Within low permeability zones, contaminants are stored as a dissolved phase in water and as a sorbed phase on or in solids. The process of contaminants moving into low permeability layers via diffusion is referred to as matrix diffusion. The significance of contaminants in low permeability layers is that they can sustain dissolved plumes in transmissive zones long after the DNAPL source is gone.[16][17]
Chlorinated solvents can also be transformed by both abiotic and biotic processes at normal groundwater temperatures, leading to the production of many intermediate chlorinated compounds in groundwater that were also of health concern. [18] PCE was first reported to be biologically reduced under anaerobic conditions to form TCE,[19] and later TCE was also found to be biologically reduced to form cis-1,2-dichloroethene (cis-DCE) and VC which is called reductive dechlorination.[20] [21] VC was of even greater concern than the parent compounds as it was a known human carcinogen. However, in 1989 VC was also found to be capable of biological reduction, forming ethene.[22] In order for biological reduction to occur, other organic compounds must be present to serve as electron donors for the bacteria. When this occurs, transformation of PCE or TCE to intermediate and end products is frequently found, leading to natural attenuation.
Transformations of TCA have been found to be even more complex as both abiotic and biotic processes are operable in its transformation.[23] Abiotically, TCA can be transformed into 1,1-dichloroethene (1,1-DCE) by removal of one chlorine atom and one hydrogen atom (dehydrohalogenation), or into acetic acid through hydrolysis reactions. The rate of transformation to acetic acid is about four times that of 1,1-DCE. Formation of 1,1-DCE is harmful as this compound is much more toxic than TCA itself, while formation of acetic acid is beneficial as this is a normal compound in the human diet and readily degraded biologically. The half-life for TCA transformation to the two different products is on the order of two years, a relatively short time when compared to the residence time of groundwater contaminants. Thus, 1,1-DCE is generally found in groundwater contaminated with TCA. TCA can also be transformed biologically by reductive dehalogenation to form 1,1-dichloroethane (1,1-DCA), which can be further reduced to chloroethane. Chloroethane can be further reduced biologically to form ethane, although chemical hydrolysis to form ethanol is generally faster. [15]
CT also can be transformed by both abiotic and biotic processes, primarily through free radical processes that lead to a variety of possible end products.[24] The abiotic processes, however, generally require the presence of a reducing agent of some type, and thus abiotic transformations do not often occur spontaneously as with TCA. Many of the CT transformation intermediates are unstable and do not last long. The main compound of concern found present from CT transformation, either abiotic or biotic, is chloroform. This too can be transformed by both abiotic and biotic processes, although generally much slower than CT.[15]
APPLICABLE REMEDIATION TECHNOLOGIES
Because of their physico-chemical properties, most chlorinated solvents are relatively recalcitrant in the subsurface, posing challenges for remediation. Fortunately, a number of ex situ and in situ approaches can be used are available to address chlorinated solvent contamination. However, the choice of which technology to implement is very site specific. It is important to thoroughly characterize the site to make a thorough evaluation and comparison of the remediation options and select an appropriate approach with a high probability of success. At a minimum, site characterization should provide information on: 1) the target chlorinated solvent(s); 2) the expected fate and transport of these contaminants based on their chemical properties; 3) the current sitewide biogeochemistry; 4) the sitewide hydrogeology and its influence on both the lateral and vertical extent of the contamination; 5) the remedial goals and objectives of the cleanup; 6) the schedule constraints; and 7) the available budget. Knowing this information, one can choose from the following list of technologies.
For Soil
Ex Situ Treatment Technologies for Soil
- Excavation and Off-site Disposal. Applicable to vadose zone (unsataturated) soils. Vertical and lateral access to the contaminated material by excavators is key for this removal approach.
- Excavation and On-site Mobile Steam Distillation. Applicable to vadose zone and saturated soils. Vertical and lateral access to the contaminated material by excavators is key for this removal approach. Soils are heated to above the boiling point of the contaminants, then contaminants are then removed through condensation, leaving the soil solvent-free. Soils can then be returned to the excavation.
In Situ Treatment Technologies for Soil
- VOS (Vadose Oil Substrate). VOS® is a technology that helps microbes breakdown chlorinated solvents in the vadose zone before they reach groundwater. VOS technology is based on emulsified oil technology used for treating groundwater, but in this approach, VOS is injected into the unsaturated soil to create a long-lasting treatment zone that promotes anaerobic reductive dechlorination. *Soil Vacuum Extraction (SVE). Applicable to vadose zone soil with moderate to high permeability. Relies on a compound’s ability to partition from where it is adsorbed to the soil into to soil gas where it can be vacuum extracted under pressure and discharged or treated before discharge.
- Vitrification. Involves applying and electrical current to the contaminated soil matrix to solidify the matrix and bind the contamination in place.
For Groundwater
Ex Situ Treatment Technologies for Groundwater
- Aggressive Fluid Vacuum Recovery (AFVR). Applicable to localized areas of groundwater containing elevated concentrations of chlorinated solvents including DNAPL. Involves short-term mechanical removal of groundwater by vacuum extraction. Contaminated groundwater is disposed off site.
- Pump and Treat (P&T) with Discharge, Air Stripping (AS) or Carbon Adsorption (CA). Typically applied where hydraulic control of contaminant migration is desirable along with contaminant removal. Discharging contaminated water or treated water to a receiving feature (e.g., stream, wasterwater treatment plant) may require a permit. Air stripping may require off-gas treatment; carbon adsorption cells must be monitored for breakthrough and replacement.
In Situ Treatment Technologies for Groundwater
- Physical Approaches (Thermal). Thermal treatment involves inserting heating probes into the contaminated aquifer with the intent of raising the temperature above the boiling point of the contaminant to drive the contamination from the aqueous phase into the vapor phase. As the contaminated vapor migration upward into the overlying unsaturated soil, it is then captured by SVE for removal.
- Chemical Approaches (In Situ Chemical Oxidation [ISCO], In Situ Chemical Reduction [ISCR]). ISCO uses strong chemical oxidizers (e.g., potassium permanganate, sodium persulfate, Fenton’s Reagent, or ozone) to destroy the contaminants in place. ISCR is the combination of abiotic chemical reduction using zero valent iron (ZVI) and/or reduced minerals coupled with anerobic bioremediation to treat chlorinated solvent contamination in groundwater.
- Biological (Enhanced Reductive Dechlorination [ERD], Co-metabolism, Monitored Natural Attenuation [MNA]). Biological approaches serve to stimulate specific native or inoculated microbial communities in the metabolic removal of chlorine from the solvent yielding non-toxic end-products.
REFERENCES
- ^ 1.0 1.1 Pankow, J.F. and Cherry, J.A., 1996. Dense Chlorinated Solvents and Other DNAPLs in Groundwater, Waterloo Press, Portland, OR. ISBN-10: 0964801418/ISBN-13: 978-0964801417
- ^ Doherty RE 2000. A history of the production and use of carbon tetrachloride, tetrachloroethylene, trichloroethylene and 1,1,1-trichloroethane in the United States: Part 1. Historical background; carbon tetrachloride and tetrachloroethylene. J Environ Forensics1:69–81
- ^ 3.0 3.1 3.2 Cwiertny, D. M. and M.M. Scherer, 2010. Chapter 2, Chlorinated Solvent Chemistry: Structures, Nomenclature and Properties. In: HF Stroo and CH.Ward (eds.). In Situ Remediation of Chlorinated Solvent Plumes. Springer, pp. 29-37. ISBN: 978-0-387-23036-8/e-ISBN: 978-0-387-23079-5, DOI: 10.1007/0-387-23079-3_32
- ^ U.S. Environmental Protection Agency (USEPA). 1996. A Citizen’s Guide to Treatment Walls. Office of Solid Waste and Emergency Response, Washington, DC, EPA 542-F-96-016, September 1996
- ^ Agency for Toxic Substance and Disease Registry (ATSDR). 2003. Trichloroethylene CAS# 79-01-6, Division of Toxicology ToxFAQs™, July 2003.
- ^ U.S. Department of Health and Human Services. 2011. Report on Carcinogens, Twelfth Edition. National Toxicology Program. June 10, 2011.
- ^ U.S. Environmental Protection Agency (USEPA). 2003. 2001 Toxics release inventory public data release report. Office of Environmental Information, Washington, D.C., USEPA 260–R–03–001, July 2003.
- ^ U.S. Environmental Protection Agency (USEPA). 2011. Toxicological Review of Trichloroethylene. National Center for Environmental Assessment, Washington, DC, EPA 635-R-09-011F, September 2011.
- ^ U.S. Environmental Protection Agency (USEPA). 2012. Toxicological Review of Tetrachloroethylene (Perchloroethylene). National Center for Environmental Assessment, Washington, DC, EPA 635-R-08-011F, February 2012.
- ^ 10.0 10.1 Agency for Toxic Substance and Disease Registry (ATSDR). 2013. 2013 Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) Priority List of Hazardous Substances.
- ^ U.S. Department of Health and Human Services. 2011. Report on Carcinogens, Twelfth Edition. National Toxicology Program. June 10, 2011.
- ^ U.S. Environmental Protection Agency (USEPA). 2011. State Coalition for Remediation of Drycleaners – Website Resources Brochure. Office of Solid Waste and Emergency Response, Washington, DC, EPA 542-F-11-009, May 2011.
- ^ U.S. Environmental Protection Agency (USEPA). 2001. Trichloroethylene Health Risk Assessment: Synthesis and Characterization, National Center for Environmental Assessment, Office of Research and Development, Washington, DC, EPA 600-P-01-002A, August 2001.
- ^ 14.0 14.1 Sale T., Newell C., Stroo H., Hinchee R., and Johnson P. 2008. Frequently Asked Questions Regarding Management of Chlorinated Solvents in Soil and Groundwater.
- ^ 15.0 15.1 15.2 McCarty P.L. 2010. Chapter 1, Gorundwater Contamination by Chlorinated Solvents: History, Remediation Technologies and Strategies. In: HF Stroo and CH.Ward (eds.). In Situ Remediation of Chlorinated Solvent Plumes. Springer, pp. 29-37. ISBN: 978-0-387-23036-8/e-ISBN: 978-0-387-23079-5, DOI: 10.1007/0-387-23079-3_32 Cite error: Invalid
<ref>tag; name "M 2010" defined multiple times with different content Cite error: Invalid<ref>tag; name "M 2010" defined multiple times with different content - ^ Air Force Center for Engineering and the Environment (AFCEE). 2007. AFCEE Source Zone Initiative, Contributing Authors: T. Sale, B. Twitchell, F. Marinelli—Colorado State University; T. Illangasekare, B. Wilking, and D. Rodriguez—Colorado School of Mines.
- ^ Chapman, S.W. and Parker, B.L., 2005. Plume Persistence Due to Aquitard Back Diffusion Following Dense Non-aqueous Phase Liquid Removal or Isolation, Water Resource Research, Vol. 41, No. 12, W12411. DOI: 10.1029/2005WR004224
- ^ Vogel TM, Criddle CS, McCarty PL. 1987. Transformations of halogenated aliphatic compounds. Environ Sci Technol 21:722–736.
- ^ Bouwer EJ, Rittmann BE, McCarty PL. 1981. Anaerobic degradation of halogenated 1- and 2-carbon organic compounds. Environ Sci Technol 15:596–599.
- ^ Parsons F, Lage BG. 1985. Chlorinated organics in simulated groundwater environments. J Am Water Works Assoc 77:52–59.
- ^ Vogel TM, McCarty PL. 1985. Biotransformation of tetrachloroethylene to trichloroethylene, dichloroethylene, vinyl chloride, and carbon dioxide under methanogenic conditions. Appl Environ Microbiol 49:1080–1083
- ^ Freedman DL, Gossett JM. 1989. Biological reductive dechlorination of tetrachloroethylene and trichloroethylene to ethylene under methanogenic conditions. Appl Environ Microbiol 55:2144–2151.
- ^ Vogel TM, McCarty PL. 1987. Abiotically 1,1,1-trichloroethane Environ Sci Technol 21:1208–1213.
- ^ Criddle CS, McCarty PL. 1991. Electrolytic model system for reductive dehalogenation in aqueous enviroments. Environ Sci Technol 25:973–978.
SEE ALSO
Add Related Pages from within this Wiki here
| Species | Formula Weight | Carbon Oxidation Statea | Density (ρ)(g/mL) | Solubility (mg/L) | Vapor Pressure (ρ0)(kPa) | Henry's Law Constant (KH)(x10-3 | Log Kow | Log Kocb | MCLc (μg/L) |
| Chlorinated Methanes | |||||||||
| tetrachloromethane | x | x | x | x | x | x | x | x | x |
| trichloromethane | x | x | x | x | x | x | x | x | x |
| dichloromethane | x | x | x | x | x | x | x | x | x |
| chloromethane | x | x | x | x | x | x | x | x | x |
| Chlorinated Ethanes | |||||||||
| hexachloroethane | x | x | x | x | x | x | x | x | x |
| pentachloroethane | x | x | x | x | x | x | x | x | x |
| 1,1,1,2-tetrachloroethane | x | x | x | x | x | x | x | x | x |
| 1,1,2,2-tetrachloroethane | x | x | x | x | x | x | x | x | x |
| 1,1,2-trichloroethane | x | x | x | x | x | x | x | x | x |
| 1,1,1-trichloroethane | x | x | x | x | x | x | x | x | x |
| 1,2-dichloroethane | x | x | x | x | x | x | x | x | x |
| 1,1-dichloroethane | x | x | x | x | x | x | x | x | x |
| chloroethane | x | x | x | x | x | x | x | x | x |
| Chlorinated Ethenes | |||||||||
| tetrachloroethene | x | x | x | x | x | x | x | x | x |
| trichloroethene | x | x | x | x | x | x | x | x | x |
| cis-1,2-dichloroethene | x | x | x | x | x | x | x | x | x |
| trans-1,2-dichloroethene | x | x | x | x | x | x | x | x | x |
| 1,1-dichloroethene | x | x | x | x | x | x | x | x | x |
| chloroethene | x | x | x | x | x | x | x | x | x |
Qualification Summary
Dr. Yuncu specializes in the application of physico-chemical treatment processes and bioremediation of hazardous compounds in soil and groundwater. She serves as a project manager and lead engineer on many of Solutions-IES’ in situ bioremediation projects. She is an author of several publications and an active presenter of in situ remediation technologies at international conferences.
Education/Training
Ph.D. - Civil, Construction & Environmental Engineering, NC State University, December 2010 M.S. - Environmental Engineering, Middle East Technical University, September 2003 B.S. - Environmental Engineering, Middle East Technical University, June 2000
Registrations/Certifications/Licenses
Professional Engineer, North Carolina, 2014
Representative Projects
Quantifying Mobile-Immobile Mass Transfer using Direct Push Tools – Strategic Environmental Research and Development Program, Department of Defense Project Manager - The overall objective of this project is to develop methods to better characterize and model the mass transfer of contaminants between higher and lower mobility zones and its impact on the long-term release of contaminants in groundwater (Funded – project will start in May 2015).
Novel Substrate Application for Bioremediation of Comingled 1,4-Dioxane and Chlorinated Solvent Plumes - Air Force Civil Engineer Center Principal Investigator - The overall objective of this project is to demonstrate (proof of concept) a simple, low-cost approach for enhancing the in situ cometabolic biodegradation of 1,4-dioxane and TCE using two-barrier system to create distinct geochemical zones (anaerobic/aerobic) within a comingled plume.
Generation of Biodegradation - Sorption Barriers for Munitions Constituents - Environmental Security Technology Certification Program, Department of Defense Project Engineer - The overall objective of this project is to develop and demonstrate a process to enhance the sorption and/or degradation of explosives and perchlorate in soils by spray application of an organic amendment solution, followed by irrigation to carry the amendments deeper into the soil profile.
Anaerobic Bioremediation of DNAPLs - Air Force Civil Engineer Center Project Engineer - The overall objective is to demonstrate enhanced dissolution and biodegradation of a chlorinated solvent DNAPL using an emulsified oil technology formulated with a slow-release pH buffer and a bioaugmentation culture.
Groundwater MNA and Landfill Monitoring, Ceiba, Puerto Rico – Naval Facilities Engineering Command Southeast
Task Manager responsible for reviewing and evaluating analytical data and report preparation - The project is a reoccurring large scale sampling event that includes the collection of groundwater, landfill gas, soil, and ambient gas samples.
- ^ Cwiertny, D. M. and M.M. Scherer, 2010. Chapter 2, Chlorinated Solvent Chemistry: Structures, Nomenclature and Properties. In: HF Stroo and CH.Ward (eds.). In Situ Remediation of Chlorinated Solvent Plumes. Springer, pp. 29-37. ISBN: 978-0-387-23036-8/e-ISBN: 978-0-387-23079-5, DOI: 10.1007/0-387-23079-3_32