Difference between revisions of "User:Debra Tabron/sandbox"
Debra Tabron (talk | contribs) |
Debra Tabron (talk | contribs) |
||
| Line 60: | Line 60: | ||
<blockquote>"With TPHCWG method, TPH is measured using an analytical method that provides somewhat more detail than total TPH concerning the composition of the petroleum mixture. These analytical methods include TCEQ1006 (based on the TPHCWG) or Massachusetts EPH/VPH. These methods provide concentration results for six (Massachusetts EPH/VPH) or 13 (TCEQ1006) different TPH fractions separated into different classes by compound type (aliphatics vs. aromatics, Figure 4) and by carbon number. These fraction results can be used in risk assessments by assigning conservative toxicity values and fate and transport characteristics to each different fraction. The toxicity and fate and transport values can be obtained from guidance documents that describe the application of the TPH fraction approach for risk assessment<ref>Massachusetts Department of Environmetnal Protection (MassDEP), M., 2002. Characterizing Risks Posed by Petroleum Contaminated Sites: Implementation of the MADEP VPH/EPH Approach. Final Policy# WSC-02-411. Commonwealth of Massachusetts Executive Office of Environmental Affairs. [[media:MassDEP-2002_VPH-EPH.pdf| Report.pdf]]</ref><ref name= "Weisman1998">Weisman, W., 1998. Analysis of Petroleum Hydrocarbons in Environmental Media, Total Petroleum Hydrocarbon Criteria Working Group Series, v.1. Amherst Scientific Publishers, Amherst, Mass. [[Media: Weisman-1998_TPH Criteria Working Group Series Vol1.pdf | Report.pdf]]</ref>. While typically used to demonstrate that soils containing petroleum hydrocarbons have heaver, low-risk fractions, the method can also be applied to groundwater samples."</blockquote>(NAVFAC 2016) | <blockquote>"With TPHCWG method, TPH is measured using an analytical method that provides somewhat more detail than total TPH concerning the composition of the petroleum mixture. These analytical methods include TCEQ1006 (based on the TPHCWG) or Massachusetts EPH/VPH. These methods provide concentration results for six (Massachusetts EPH/VPH) or 13 (TCEQ1006) different TPH fractions separated into different classes by compound type (aliphatics vs. aromatics, Figure 4) and by carbon number. These fraction results can be used in risk assessments by assigning conservative toxicity values and fate and transport characteristics to each different fraction. The toxicity and fate and transport values can be obtained from guidance documents that describe the application of the TPH fraction approach for risk assessment<ref>Massachusetts Department of Environmetnal Protection (MassDEP), M., 2002. Characterizing Risks Posed by Petroleum Contaminated Sites: Implementation of the MADEP VPH/EPH Approach. Final Policy# WSC-02-411. Commonwealth of Massachusetts Executive Office of Environmental Affairs. [[media:MassDEP-2002_VPH-EPH.pdf| Report.pdf]]</ref><ref name= "Weisman1998">Weisman, W., 1998. Analysis of Petroleum Hydrocarbons in Environmental Media, Total Petroleum Hydrocarbon Criteria Working Group Series, v.1. Amherst Scientific Publishers, Amherst, Mass. [[Media: Weisman-1998_TPH Criteria Working Group Series Vol1.pdf | Report.pdf]]</ref>. While typically used to demonstrate that soils containing petroleum hydrocarbons have heaver, low-risk fractions, the method can also be applied to groundwater samples."</blockquote>(NAVFAC 2016) | ||
| + | ==Physical and Chemical Properties== | ||
| + | PHCs are generally divided into two groups: [[wikipedia: Aliphatic compound | aliphatics]] and [[Wikipedia: Aromaticity | aromatics]]. Aliphatics include: a) alkanes that contain single bonds between carbon atoms and have formulas of CnH2n+2, b) alkenes, which contain one or more double bonds between carbon atoms and have formulas of CnH2n, and c) cycloalkanes, which contain single-bonded carbon atoms in cyclic structures. Aromatics have one or more benzene rings as part of their structure. Monoaromatics have one benzene ring as part of their structure while polycyclic aromatic hydrocarbons (PAHs) contain two or more bonded benzene rings. Table 1 summarizes the physical and chemical properties of selected PHCs and fuel additives. | ||
| + | {| class="wikitable mw-collapsible" style="text-align:center;" | ||
| + | |+ Table 1. Nomenclature, Structure, Chemical and Physical Properties of Selected Petroleum Hydrocarbons and Fuel Additives. | ||
| + | |- | ||
| + | ! IUPAC Name | ||
| + | ! Common Name</br>(Acronym) | ||
| + | ! Molecular Formula | ||
| + | ! Chemical Structure | ||
| + | ! Formula Weight</br><small>(g/mol)</small> | ||
| + | ! Density</br><small>(g/mL)</small> | ||
| + | ! Solubility</br><small>(mg/L at 20° to 25°C)</small> | ||
| + | ! Vapor Pressure</br><small>(mmHg)</small> | ||
| + | ! Henry’s Law Constant</br><small>(Dimensionless)</small> | ||
| + | ! Log ''K<sub>ow</sub>''<ref name="ATSDR 2019"> Agency for Toxic Substance and Disease Registry (ATSDR), 2019. Priority List of Hazardous Substances. US Department of Health and Human Services. https://www.atsdr.cdc.gov/spl Searchable Toxic Substances Portal: https://www.atsdr.cdc.gov/substances/index.asp </ref> | ||
| + | ! MCL<ref name="USEPA 2019"> US Environmental Protection Agency (USEPA),2019. National Primary Drinking Water Regulations https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations </ref></br><small>(mg/L)</small> | ||
| + | |- | ||
| + | | colspan="11" style="background-color:white;" | '''Aromatic Hydrocarbons''' | ||
| + | |- | ||
| + | | style="text-align:left;" | Benzene || Benzene || C<sub>6</sub>H<sub>6</sub> || [[File:Yuncu1w2Benzene.png | 70px]] || 7.81 || 0.88 || 1,750<ref name="USEPA 1996"> US Environmental Protection Agency (USEPA), 1996. Soil screening guidance: User’s guide. U.S. Environmental Protection Agency, Office of Solid Waste and Emergency Response, Washington, D.C. Publication 9355.4-23. https://rais.ornl.gov/documents/SSG_nonrad_user.pdf </ref> || 76<ref name="Howard 1989"> Howard, P.H., 1989. Handbook of Environmental Fate and Exposure Data for Organic Chemicals, Vol. 1 - Large Production and Priority Pollutants. Lewis Publishers, Inc. Chelsea, Michigan.</ref> || 0.228<ref name="USEPA 1996" /> || 2.13 || 0.005 | ||
| + | |- | ||
| + | | style="text-align:left;" | Ethylbenzene|| Ethylbenzene || C<sub>8</sub>H<sub>10</sub> || [[File:Yuncu1w2Ethylbenzene.png | 70px]] || 106.2 || 0.87 || 169<ref name="USEPA 1996" /> || 7<ref name="Howard 1989" /> || 0.323<ref name="USEPA 1996" /> || 4.34 || 0.7 | ||
| + | |- | ||
| + | | style="text-align:left;" | Methylbenzene || Toluene || C<sub>7</sub>H<sub>8</sub> || [[File:Yuncu1w2Methylbenzene.png | 40px]] || 92.1 || 0.87 || 526<ref name="USEPA 1996" />|| 22<ref name="Howard 1989" /> || 0.272<ref name="USEPA 1996" /> || 2.73 || 1 | ||
| + | |- | ||
| + | | style="text-align:left;" | Total xylenes* || -- || -- || -- || -- || -- || -- || -- || -- || -- || 10 | ||
| + | |- | ||
| + | | style="text-align:right;" | 1,3-Dimethylbenzene || m-Xylene || C<sub>8</sub>H<sub>10</sub> || [[File:Yuncu1w2_13Dimethylbenzene.png | 70px]] || 106.2 || 0.86 || 161<ref name="USEPA 1996" /> || 6<ref name="Howard 1989" /> || 0.301<ref name="USEPA 1996" /> || 3.20 || -- | ||
| + | |- | ||
| + | | style="text-align:right;" | 1,2-Dimethylbenzene || o-Xylene || C<sub>8</sub>H<sub>10</sub> || [[File:Yuncu1w2_12Dimethylbenzene.png | 70px]] || 106.2 || 0.88 || 178<ref name="USEPA 1996" /> || 5<ref name="Howard 1989" /> || 0.213<ref name="USEPA 1996" /> || 3.12 || -- | ||
| + | |- | ||
| + | | style="text-align:right;" | 1,4-Dimethylbenzene || p-Xylene || C<sub>8</sub>H<sub>10</sub> || [[File:Yuncu1w2_14Dimethylbenzene.png | 90px]] || 106.2 || 0.86 || 185<ref name="USEPA 1996" />|| 6.5<ref name="Howard 1989" /> || 0.314<ref name="USEPA 1996" /> || 3.15 || -- | ||
| + | |- | ||
| + | | colspan="11" style="background-color:white;" | '''Polycyclic Aromatic Hydrocarbons''' | ||
| + | |- | ||
| + | | style="text-align:left;" | 1,2-Dihydroacenaphthylene || Acenaphthene || C<sub>12</sub>H<sub>10</sub> || [[File:Yuncu1w2_12Dihydroacenaphthylene.png | 70px]] || 154.2 || 1.22 || 4.24<ref name="USEPA 1996" />|| 4.47x10<sup>-3</sup><ref name="ATSDR 2019" /> || 6.36x10<sup>-3</sup><ref name="USEPA 1996" /> || 3.98 || -- | ||
| + | |- | ||
| + | | style="text-align:left;" | Acenaphthylene || Acenaphthylene || C<sub>12</sub>H<sub>8</sub> || [[File:Yuncu1w2Acenaphthylene.png | 70px]] || 152.2 || 0.90 || 3.93<ref name="ATSDR 2019" />|| 0.029<ref name="ATSDR 2019" /> || 0.0593<ref name="ATSDR 2019" /> || 4.07 || -- | ||
| + | |- | ||
| + | | style="text-align:left;" | Anthracene || Anthracene || C<sub>14</sub>H<sub>10</sub> || [[File:Yuncu1w2Anthracene.png | 70px]] || 178.2 || 1.28 || 0.0434<ref name="USEPA 1996" /> || 1.7x10<sup>-5</sup><ref name="ATSDR 2019" /> || 2.67x10<sup>-3</sup><ref name="USEPA 1996" /> || 4.45 || -- | ||
| + | |- | ||
| + | | style="text-align:left;" | Benz[a]anthracene || Benz[a]anthracene || C<sub>18</sub>H<sub>12</sub> || [[File:Yuncu1w2BenzAanthracene.png | 70px]] || 228.3 || 1.27 || 9.4x10<sup>-3</sup><ref name="USEPA 1996" />|| 2.2x10<sup>-8</sup><ref name="ATSDR 2019" /> || 1.4x10<sup>-4</sup><ref name="USEPA 1996" /> || 5.61 || -- | ||
| + | |- | ||
| + | | style="text-align:left;" | Benzo[a]pyrene || Benzo[a]pyrene || C<sub>20</sub>H<sub>12</sub> || [[File:Yuncu1w2BenzoApyrene.png | 70px]] || 252.3 || 1.35 || 1.6x10<sup>-3</sup><ref name="USEPA 1996" /> || 5.6x10<sup>-9</sup><ref name="ATSDR 2019" /> || 4.63x10<sup>-5</sup><ref name="USEPA 1996" /> || 6.06 || 2x10<sup>-4</sup> | ||
| + | |- | ||
| + | | style="text-align:left;" | Benzo[b]fluoranthene || Benzo[b]fluoranthene || C<sub>20</sub>H<sub>12</sub> || [[File:Yuncu1w2BenzoBfluoranthene.png | 70px]] || 252.3 || 1.29 || 1.5x10<sup>-3</sup><ref name="USEPA 1996" /> || 5.0x10<sup>-7</sup><ref name="ATSDR 2019" /> || 4.55x10<sup>-3</sup><ref name="USEPA 1996" /> || 6.04 || -- | ||
| + | |- | ||
| + | | style="text-align:left;" | Benzo[g,h,i]perylene || Benzo[g,h,i]perylene || C<sub>22</sub>H<sub>12</sub> || [[File:Yuncu1w2BenzoGHIperylene.png | 70px]] || 276.3 || 1.38 || 2.6x10<sup>-4</sup><ref name="ATSDR 2019" /> || 1.0x10<sup>-10</sup><ref name="ATSDR 2019" /> || 5.89x10<sup>-6</sup><ref name="ATSDR 2019" /> || 6.50 || -- | ||
| + | |- | ||
| + | | style="text-align:left;" | Benzo[k]fluoranthene || Benzo[k]fluoranthene || C<sub>20</sub>H<sub>12</sub> || [[File:Yuncu1w2BenzoKfluoranthene.png | 70px]] || 252.3 || 1.29 || 8x10<sup>-4</sup><ref name="USEPA 1996" /> || 9.6x10<sup>-11</sup><ref name="ATSDR 2019" /> || 3.4x10<sup>-5</sup><ref name="USEPA 1996" /> || 6.06 || -- | ||
| + | |- | ||
| + | | style="text-align:left;" | Chrysene || Chrysene || C<sub>18</sub>H<sub>12</sub> || [[File:Yuncu1w2Chrysene.png | 70px]] || 228.3 || 1.27 || 1.6x10<sup>-3</sup><ref name="USEPA 1996" /> || 6.3x10<sup>-7</sup><ref name="ATSDR 2019" /> || 3.88x10<sup>-3</sup><ref name="USEPA 1996" /> || 5.16 || -- | ||
| + | |- | ||
| + | | style="text-align:left;" | Fluoranthene || Fluoranthene || C<sub>16</sub>H<sub>10</sub> || [[File:Yuncu1w2Fluoranthene.png | 70px]] || 202.3 || 1.25 || 0.206<ref name="USEPA 1996" />|| 5.0x10<sup>-6</sup><ref name="ATSDR 2019" /> || 6.6x10<sup>-4</sup><ref name="USEPA 1996" /> || 4.90 || -- | ||
| + | |- | ||
| + | | style="text-align:left;" | 9''H''-Fluorene || Fluorene || C<sub>13</sub>H<sub>10</sub> || [[File:Yuncu1w2_9hFluorene.png | 70px]] || 166.2 || 1.20 || 1.98<ref name="USEPA 1996" /> || 3.2x10<sup>-4</sup><ref name="ATSDR 2019" />|| 2.61x10<sup>-3</sup><ref name="USEPA 1996" /> || 4.18 || -- | ||
| + | |- | ||
| + | | style="text-align:left;" | Indeno[1,2,3-c,d]pyrene || Indeno[1,2,3-c,d]pyrene || C<sub>22</sub>H<sub>12</sub> || [[File:Yuncu1w2Indeno123CDpyrene.png | 70px]] || 276.4 || 1.40 || 2.2x10<sup>-5</sup><ref name="USEPA 1996" /> || ~10<sup>-11</sup>to 10<sup>-6</sup><ref name="ATSDR 2019" /> || 7.0x10<sup>-5</sup><ref name="USEPA 1996" /> || 6.58 || -- | ||
| + | |- | ||
| + | | style="text-align:left;" | Naphthalene || Naphthalene || C<sub>10</sub>H<sub>8</sub> || [[File:Yuncu1w2Naphthalene.png | 70px]] || 128.2 || 1.16 || 31<ref name="USEPA 1996" />|| 0.087<ref name="ATSDR 2019" /> || 0.0198<ref name="USEPA 1996" />|| 3.29 || -- | ||
| + | |- | ||
| + | | style="text-align:left;" | Phenanthrene || Phenanthrene || C<sub>14</sub>H<sub>10</sub> || [[File:Yuncu1w2Phenanthrene.png | 70px]] || 178.2 || 1.18 || 1.20<ref name="ATSDR 2019" /> || 6.8x10<sup>-4</sup><ref name="ATSDR 2019" /> || 1.05x10<sup>-3</sup><ref name="ATSDR 2019" /> || 4.45 || -- | ||
| + | |- | ||
| + | | style="text-align:left;" | Pyrene || Pyrene || C<sub>16</sub>H<sub>10</sub> || [[File:Yuncu1w2Pyrene.png | 70px]] || 202.3 || 1.27 || 0.135<ref name="USEPA 1996" />|| 2.5x10<sup>-6</sup><ref name="ATSDR 2019" />|| 4.5x10<sup>-4</sup><ref name="USEPA 1996" />|| 4.88 || -- | ||
| + | |- | ||
| + | | colspan="11" style="background-color:white;" | '''Fuel Additives''' | ||
| + | |- | ||
| + | | style="text-align:left;" | 2-Methoxy-2-methylpropane || Methyl ''tert''-butyl ether (MTBE) || C<sub>5</sub>H<sub>12</sub>O || [[File:Yuncu1w2MTBE.png | 70px]] || 88.2 || 0.74 || 51,000<ref> National Center for Biotechnology Information (NCBI), 2020. PubChem Database. Methyl tert-butyl ether, CID=15413. https://pubchem.ncbi.nlm.nih.gov/compound/Methyl-tert-butyl-ether </ref> || 245 to 256<ref name="NSTC 1997"> National Science and Technology Council (NSTC), 1997. Interagency Assessment of Oxygenated Fuels. Committee on Environment and Natural Resources. [[media: NSTC-1997_Interagency_Assessment_of_Oxygenated_Fuels.pdf | Report.pdf]]</ref> || 0.123 to 0.024<ref name="NSTC 1997" /> || 1.20<ref name="NSTC 1997" /> || -- | ||
| + | |- | ||
| + | | style="text-align:left;" | 2-Methylpropan-2-ol || ''tert''-Butyl alcohol (TBA) || C<sub>4</sub>H<sub>10</sub>O || [[File:Yuncu1w2TBA.png | 70px]] || 74.12 || 0.78 || Infinite<ref name="NSTC 1997" />|| 40 to 42<ref name="NSTC 1997" /> || 4.3x10<sup>-4</sup> to 5.9x10<sup>-4</sup><ref name="NSTC 1997" />|| 0.35<ref name="NSTC 1997" /> || -- | ||
| + | |- | ||
| + | | colspan="11" style="text-align:left; background-color:white;" | Notes:</br>''K<sub>ow</sub>'' is the Octanol/Water partition coefficient (dimensionless).</br>MCL is the Maximum Contaminant Level.</br>* Total xylenes consist of three different chemical isomers (meta-xylene, ortho-xylene and para-xylene) but are typically regulated as total xylenes. | ||
| + | |||
| + | <sup>a</sup> Source: USEPA 1996<ref>US Environmental Protection Agency (USEPA), 1996. Soil screening guidance: User’s guide. Office of Solid Waste and Emergency Response, Washington, D.C., Publication 9355.4-23. [[media:USEPA-1996 Soil screening guidance.pdf| Report.pdf]]</ref></br> | ||
| + | <sup>b</sup> Source: Howard 1989<ref>Howard, P.H., Jarvis, W.F., Sage, G.W., Basu, D.K., Gray, D.A., Meylan, W. and Crosbie, E.K., 1989. Handbook of Environmental Fate and Exposure Data for Organic Chemicals, Volume 1, Large Production and Priority Pollutants. ISBN: 9780873711517 - CAT# L151</ref></br> | ||
| + | <sup>c</sup> [https://www.atsdr.cdc.gov/ Source: ATSDR, 2017]</br> | ||
| + | <sup>d</sup> Source https://www.epa.gov/dwstandardsregulations#List (USEPA 2019) – Cannot find link source</br> | ||
| + | <sup>e</sup> Source: NCBI, 2020<ref>National Center for Biotechnology Information (NCBI), 2020. Methyl tert-butyl ether, PubChem CID=15413. National Institutes of Health, PubChem Database [https://pubchem.ncbi.nlm.nih.gov/compound/Methyl-tert-butyl-ether] </ref></br> | ||
| + | <sup>f</sup> Source: NSTC 1997<ref>National Science and Technology Council, 1997. Interagency assessment of oxygenated fuels. National Science and Technology Committee, Committee on Environment and Natural Resources. [[media:NSTC-1997_Interagency_Assessment_of_Oxygenated_Fuels.pdf| Report.pdf]]</ref> | ||
Revision as of 20:13, 14 April 2020
Petroleum hydrocarbon (PHC) contamination is one of the most common environmental issues encountered by environmental professionals. Environmental pollution caused by releases of petroleum to land, surface water, or the subsurface is of concern because chemicals in PHCs can present a risk to human and environmental receptors if concentrations in environmental media are high enough. A variety of remediation technologies have been developed over the years to reduce the concentrations of petroleum hydrocarbon contaminants in soil and groundwater. However, the complete restoration of sites with petroleum contamination in soils and groundwater is challenging because 1) PHCs in the form of light non-aqueous phase liquids (LNAPLs) can become trapped in soil pores as an immobile, residual phase; and 2) some of the chemical compounds in LNAPL can transfer out of the residual LNAPL and migrate along potential exposure pathways in groundwater, soil, sediment, and air. Fortunately, most PHC constituents can biodegrade either in aerobic or anaerobic environments, making PHC contaminated sites somewhat easier to remediate than typical chlorinated solvents or metals contaminated sites.
Related Article(s):
- Polycyclic Aromatic Hydrocarbons (PAHs)
- Monitored Natural Attenuation (MNA) of Fuels
- Sorption of Organic Contaminants
- Natural Source Zone Depletion (NSZD)
- LNAPL Remediation Technologies (Coming soon)
- NAPL Mobility
- LNAPL Conceptual Site Model (Coming soon)
- Natural Attenuation in Source Zone and Groundwater Plume - Bemidji Crude Oil Spill
CONTRIBUTOR(S): Dr. Bilgen Yuncu
Key Resource(s):
- Characteristics of Dissolved Petroleum Hydrocarbon Plumes[1]
- Evaluating LNAPL Remedial Technologies for Achieving Project Goals[2]
- LNAPL-3: LNAPL Site Management: LCSM Evolution, Decision Process, and Remedial Technologies[3]
- New Developments in LNAPL Site Management[4]
- Managing Risk at LNAPL Sites[5]
Introduction
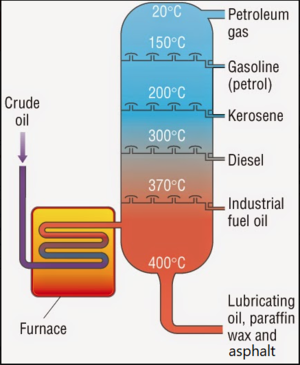
Petroleum hydrocarbons (PHCs) are the primary constituents in crude oil, gasoline (Figure 1), diesel (Figure 2), and a variety of solvents and penetrating oils. Crude Oil consists of hydrocarbon molecules extracted from the ground and transformed in petroleum (oil) refineries into petroleum products, such as gasoline, diesel fuel, asphalt base, heating oil, kerosene, and liquefied petroleum gas. Fractional distillation is used to separate the various compounds of crude oil based on the boiling points of each fraction (Figure 3). The fractions at the bottom of the fractionating column have higher boiling points than at the top. The fractions with higher boiling points are darker in color, more viscous and have less branched alkanes with more carbon atoms and higher molecular weights.
The main classes of PHCs of environmental concern are aromatic hydrocarbons (i.e., benzene, ethylbenzene, toluene, and xylenes), polycyclic aromatic hydrocarbons (PAHs; e.g. anthracene, phenanthrene and benzo[a]pyrene), gasoline additives (e.g., Methyl tert-butyl ether (MTBE), tert-Butyl alcohol (tBA)), and combustion emissions from fuels (e.g., carbon monoxide, acetaldehyde, formaldehyde, and diesel particulates).
Aromatic Hydrocarbons
Aromatic hydrocarbons contain one or more benzene rings. The name of this class comes from the fact that many of them have strong, pungent aromas. Key aromatic hydrocarbons of environmental interest are benzene, ethylbenzene, toluene, and xylenes (BTEX). BTEX compounds are the most common aromatic compounds in petroleum[6]. They are extracted from complex mixtures obtained by the refining of oil or by distillation of coal tar and are used to produce a range of important chemicals, polymers, and consumer products such as paints and lacquers, thinners, rubber products, adhesives, inks, cosmetics and pharmaceutical products. Since BTEX compounds are found naturally in crude oil, coal, and gas deposits, they can be present naturally in groundwater near these deposits.
Benzene is a known human carcinogen and therefore included in risk Group 1 of the International Agency for Research on Cancer (IARC). Long-term exposure to benzene can cause cancer in blood forming organs (i.e. leukemia)[7]. Ethylbenzene is included in risk Group 2B which consists of chemicals considered possibly carcinogenic to humans[8]. Toluene and xylenes are categorized as not classifiable as to human carcinogenicity (Group 3) by both the U.S. Environmental Protection Agency[9][10] and IARC[11], reflecting the lack of evidence for the carcinogenicity of these two chemicals.
Acute (short-term) exposure to BTEX has been associated with skin and sensory irritation, central nervous system problems (tiredness, dizziness, headache, loss of coordination), and effects on the respiratory system (eye and nose irritation). Prolonged (chronic) exposure to BTEX compounds can affect the kidney, liver, and blood systems. Surface water concentrations of BTEX compounds above 1 mg/L can produce acute toxic effects in organisms such as algae and fish.
Polycyclic Aromatic Hydrocarbons
Polycyclic aromatic hydrocarbons (PAHs) are a group of more than 100 different chemicals that are released from burning coal, oil, gasoline, trash, tobacco, wood, or other organic substances such as charcoal-broiled meat. While PAHs occur naturally in crude oil, and smoke and ash from forest fires, they are most often found as products of incomplete combustion, especially from incinerators. PAHs are often found at facilities formerly involved in creosote, coking, and wood preservative production, as well as at former manufactured gas plants that used coal as a feedstock. Most regulations, analyses, and data reporting focus on only a limited number of PAHs, typically between 14 and 20 individual PAH compounds such as: acenaphthene, acenaphthylene, anthracene, benz[a]anthracene, benzo[a]pyrene, benzo[e]pyrene, benzo[b]fluoranthene, benzo[g,h,i]perylene, benzo[j]fluoranthene, benzo[k]fluoranthene, chrysene, dibenz[a,h]anthracene, fluoranthene, fluorene, indeno[1,2,3-c,d]pyrene, naphthalene, phenanthrene and pyrene[12].
Of the more than 100 forms of PAHs, 15 are listed as "reasonably anticipated to be human carcinogens" in the 14th Report on Carcinogens[13] since exposure to these PAHs is linked to lung, liver, and skin cancers. Workers who have been exposed to large amounts of naphthalene from skin contact with the liquid form and from breathing naphthalene vapor have developed blood and liver abnormalities. PAHs are often important contaminants of concern because of their chemical and toxicological properties. Composed of multiple aromatic rings, PAHs tend to be more immobile and more persistent in the environment than the BTEX compounds, with relatively higher bioaccumulation rates. Usually, the two most important PAH compounds at PHC contamination sites are naphthalene, an IARC Group 2B possible carcinogen which is more mobile than other PAHs and typically occurs at higher concentrations, and benzo[a]pyrene, a Group 1 known carcinogen, due to its low threshold of risk to human receptors.
Gasoline (Fuel) Additives
Methyl tert-butyl ether (MTBE) is a gasoline additive used as an oxygenate (raises the oxygen content) to improve combustion of the gasoline and reduce emissions. Although MTBE was effective in reducing emissions from automobile engines, it became a problem when accidently released from leaking underground tanks to groundwater or emitted from two-cycle boat engines to surface water. Key concerns with MTBE releases include the potential to leave an unpleasant taste and odor in water containing MTBE if at high enough concentrations, and potential health risks associated with exposure to MTBE above certain concentration thresholds. MTBE has been identified as a potential human carcinogen by USEPA at high doses[14]. Human exposure to high levels of MTBE in air can also cause irritation of the eyes and respiratory tract, and effects on the central nervous system. Since 2005, the use of MTBE in gasoline has been phased out in the United States. However, groundwater in some areas of the country might still contain MTBE, and it is still being used as a gasoline additive in other parts of the world[14]. Tert-Butyl alcohol (tBA) is another fuel oxygenate, but it is also used in perfumes, as a solvent for pharmaceuticals, and as a paint remover. While MTBE may contain a small percentage of tBA, tBA is also a well‐established biodegradation by-product of MTBE and often found as a groundwater co-contaminant at gasoline-impacted sites[15]. Like MTBE, inhalation of high concentrations of tBA for prolonged exposures may produce transient effects on the central nervous system, as well as eye and mucous membrane irritation[16].
Total Petroleum Hydrocarbons
Total petroleum hydrocarbons (TPH) is a term used to describe any mixture of hydrocarbons that are found in crude oil. There are several hundreds of these compounds, but not all occur in any one sample. Because there are so many different chemicals in crude oil and in other petroleum products, it is not practical to measure each one separately. However, it is useful to measure the total amount of petroleum hydrocarbons at a site. TPH can be evaluated in several ways:
- TPH for gasoline range organics (GRO) includes hydrocarbons with 6 to 10 carbon atoms (C6-C10),
- TPH for diesel range organics (DRO) includes hydrocarbons with 10 to 28 carbons (C10-C28), and
- TPH for oil or residual range organics (ORO, RRO) includes hydrocarbons with 28 to 36 carbons (C28-C36).
- Volatile petroleum hydrocarbons (VPH) include C5-C12 aliphatics, BTEX, MTBE, naphthalene, and C9-C10 aromatics.
- Extractable petroleum hydrocarbons (EPH) include C9-C36 aliphatics and C11-C22 aromatics[6].
Because TPH represents a mixture of a large number of chemical compounds, it indicates the amount of petroleum that may be present in a sample but does not provide a direct indication of risk to human health or the environment[18][19]. For example, two soil samples impacted by equal concentrations of either baby oil or gasoline would have virtually identical TPH values, but very different toxicities. The TPH Criteria Working Group (TPHCWG) developed the TPH Fraction Method to calculate risk associated with petroleum hydrocarbon mixtures. The Naval Facilities Engineering Command (NAVFAC) (2016) described the method for applying risk to TPH measurements this way:
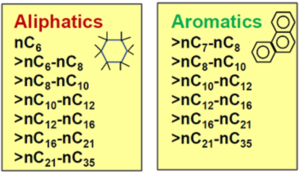
"With TPHCWG method, TPH is measured using an analytical method that provides somewhat more detail than total TPH concerning the composition of the petroleum mixture. These analytical methods include TCEQ1006 (based on the TPHCWG) or Massachusetts EPH/VPH. These methods provide concentration results for six (Massachusetts EPH/VPH) or 13 (TCEQ1006) different TPH fractions separated into different classes by compound type (aliphatics vs. aromatics, Figure 4) and by carbon number. These fraction results can be used in risk assessments by assigning conservative toxicity values and fate and transport characteristics to each different fraction. The toxicity and fate and transport values can be obtained from guidance documents that describe the application of the TPH fraction approach for risk assessment[20][21]. While typically used to demonstrate that soils containing petroleum hydrocarbons have heaver, low-risk fractions, the method can also be applied to groundwater samples."
(NAVFAC 2016)
Physical and Chemical Properties
PHCs are generally divided into two groups: aliphatics and aromatics. Aliphatics include: a) alkanes that contain single bonds between carbon atoms and have formulas of CnH2n+2, b) alkenes, which contain one or more double bonds between carbon atoms and have formulas of CnH2n, and c) cycloalkanes, which contain single-bonded carbon atoms in cyclic structures. Aromatics have one or more benzene rings as part of their structure. Monoaromatics have one benzene ring as part of their structure while polycyclic aromatic hydrocarbons (PAHs) contain two or more bonded benzene rings. Table 1 summarizes the physical and chemical properties of selected PHCs and fuel additives.
| IUPAC Name | Common Name (Acronym) |
Molecular Formula | Chemical Structure | Formula Weight (g/mol) |
Density (g/mL) |
Solubility (mg/L at 20° to 25°C) |
Vapor Pressure (mmHg) |
Henry’s Law Constant (Dimensionless) |
Log Kow[22] | MCL[23] (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| Aromatic Hydrocarbons | ||||||||||
| Benzene | Benzene | C6H6 | 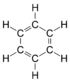 |
7.81 | 0.88 | 1,750[24] | 76[25] | 0.228[24] | 2.13 | 0.005 |
| Ethylbenzene | Ethylbenzene | C8H10 | 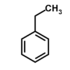 |
106.2 | 0.87 | 169[24] | 7[25] | 0.323[24] | 4.34 | 0.7 |
| Methylbenzene | Toluene | C7H8 | 92.1 | 0.87 | 526[24] | 22[25] | 0.272[24] | 2.73 | 1 | |
| Total xylenes* | -- | -- | -- | -- | -- | -- | -- | -- | -- | 10 |
| 1,3-Dimethylbenzene | m-Xylene | C8H10 | 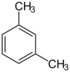 |
106.2 | 0.86 | 161[24] | 6[25] | 0.301[24] | 3.20 | -- |
| 1,2-Dimethylbenzene | o-Xylene | C8H10 | 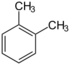 |
106.2 | 0.88 | 178[24] | 5[25] | 0.213[24] | 3.12 | -- |
| 1,4-Dimethylbenzene | p-Xylene | C8H10 | 106.2 | 0.86 | 185[24] | 6.5[25] | 0.314[24] | 3.15 | -- | |
| Polycyclic Aromatic Hydrocarbons | ||||||||||
| 1,2-Dihydroacenaphthylene | Acenaphthene | C12H10 | 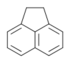 |
154.2 | 1.22 | 4.24[24] | 4.47x10-3[22] | 6.36x10-3[24] | 3.98 | -- |
| Acenaphthylene | Acenaphthylene | C12H8 | 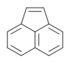 |
152.2 | 0.90 | 3.93[22] | 0.029[22] | 0.0593[22] | 4.07 | -- |
| Anthracene | Anthracene | C14H10 | 178.2 | 1.28 | 0.0434[24] | 1.7x10-5[22] | 2.67x10-3[24] | 4.45 | -- | |
| Benz[a]anthracene | Benz[a]anthracene | C18H12 | 228.3 | 1.27 | 9.4x10-3[24] | 2.2x10-8[22] | 1.4x10-4[24] | 5.61 | -- | |
| Benzo[a]pyrene | Benzo[a]pyrene | C20H12 | 252.3 | 1.35 | 1.6x10-3[24] | 5.6x10-9[22] | 4.63x10-5[24] | 6.06 | 2x10-4 | |
| Benzo[b]fluoranthene | Benzo[b]fluoranthene | C20H12 | 252.3 | 1.29 | 1.5x10-3[24] | 5.0x10-7[22] | 4.55x10-3[24] | 6.04 | -- | |
| Benzo[g,h,i]perylene | Benzo[g,h,i]perylene | C22H12 | 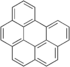 |
276.3 | 1.38 | 2.6x10-4[22] | 1.0x10-10[22] | 5.89x10-6[22] | 6.50 | -- |
| Benzo[k]fluoranthene | Benzo[k]fluoranthene | C20H12 | 252.3 | 1.29 | 8x10-4[24] | 9.6x10-11[22] | 3.4x10-5[24] | 6.06 | -- | |
| Chrysene | Chrysene | C18H12 | 228.3 | 1.27 | 1.6x10-3[24] | 6.3x10-7[22] | 3.88x10-3[24] | 5.16 | -- | |
| Fluoranthene | Fluoranthene | C16H10 | 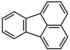 |
202.3 | 1.25 | 0.206[24] | 5.0x10-6[22] | 6.6x10-4[24] | 4.90 | -- |
| 9H-Fluorene | Fluorene | C13H10 | 166.2 | 1.20 | 1.98[24] | 3.2x10-4[22] | 2.61x10-3[24] | 4.18 | -- | |
| Indeno[1,2,3-c,d]pyrene | Indeno[1,2,3-c,d]pyrene | C22H12 | 276.4 | 1.40 | 2.2x10-5[24] | ~10-11to 10-6[22] | 7.0x10-5[24] | 6.58 | -- | |
| Naphthalene | Naphthalene | C10H8 | 128.2 | 1.16 | 31[24] | 0.087[22] | 0.0198[24] | 3.29 | -- | |
| Phenanthrene | Phenanthrene | C14H10 | 178.2 | 1.18 | 1.20[22] | 6.8x10-4[22] | 1.05x10-3[22] | 4.45 | -- | |
| Pyrene | Pyrene | C16H10 | 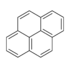 |
202.3 | 1.27 | 0.135[24] | 2.5x10-6[22] | 4.5x10-4[24] | 4.88 | -- |
| Fuel Additives | ||||||||||
| 2-Methoxy-2-methylpropane | Methyl tert-butyl ether (MTBE) | C5H12O | 88.2 | 0.74 | 51,000[26] | 245 to 256[27] | 0.123 to 0.024[27] | 1.20[27] | -- | |
| 2-Methylpropan-2-ol | tert-Butyl alcohol (TBA) | C4H10O | 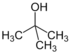 |
74.12 | 0.78 | Infinite[27] | 40 to 42[27] | 4.3x10-4 to 5.9x10-4[27] | 0.35[27] | -- |
| Notes: Kow is the Octanol/Water partition coefficient (dimensionless). MCL is the Maximum Contaminant Level. * Total xylenes consist of three different chemical isomers (meta-xylene, ortho-xylene and para-xylene) but are typically regulated as total xylenes. a Source: USEPA 1996[28]
References
See Also |
||||||||||