|
|
| Line 1: |
Line 1: |
| − | 1,4-Dioxane (14D) is a heterocyclic synthetic organic chemical that contains two ether bonds, is miscible with water, does not strongly sorb to natural or engineered materials, and does not biodegrade in many environments, all of which results in its persistence and rapid transport in aqueous environments. 14D is a suspected human carcinogen, which has resulted in relatively strict standards for drinking water sources. Advanced oxidation processes (AOPs) are the most well-developed technologies for removal of 14D from potable water supplies. Several treatment technologies are being developed for ''in situ'' treatment of 14D including chemical oxidation, cometabolic bioremediation, and thermally enhanced soil vapor extraction.
| + | The term “vapor intrusion” or “VI” refers to the migration of volatile organic compounds (VOCs) from a subsurface source into an overlying building. Preferential pathways for VI are specific migration routes that support higher contaminant flux, or discharge, into a building compared to transport through bulk soil. Sewers and other utility conduits or tunnels are an important type of preferential pathway that can result in VI when VOC vapors migrate through the interior of the conduits into buildings. |
| − | <div style="float:right;margin:0 0 2em 2em;">__TOC__</div>
| |
| | | | |
| | '''Related Article(s):''' | | '''Related Article(s):''' |
| − | *[[Biodegradation – 1,4-Dioxane]] | + | *[[Vapor Intrusion]] |
| − | *[[Biodegradation - Cometabolic]]
| |
| | | | |
| | '''CONTRIBUTOR(S):''' [[Matthew Zenker]], [[Dr. Shaily Mahendra]], and [[Dr. Michael Hyman]] | | '''CONTRIBUTOR(S):''' [[Matthew Zenker]], [[Dr. Shaily Mahendra]], and [[Dr. Michael Hyman]] |
| Line 10: |
Line 8: |
| | | | |
| | '''Key Resource(s)''': | | '''Key Resource(s)''': |
| − | *[https://doi.org/10.1201/EBK1566706629 Environmental Investigation and Remediation: 1,4-Dioxane and other solvent stabilizers]<ref name= "Mohr2010">Mohr, T.K., Stickney, J.A. and DiGuiseppi, W.H., 2010. Environmental investigation and remediation: 1, 4-dioxane and other solvent stabilizers. CRC Press. Boca Raton. 550 pages. [https://doi.org/10.1201/EBK1566706629 doi: 10.1201/EBK1566706629]</ref> | + | *[[media:2018b-McHugh-ER-201505_Conceptual_Model.pdf| Conceptual Model for Sewer/Utility Tunnel VI]]<ref>McHugh, T.; Beckley, L, 2018(b). Conceptual Model: Sewers and Utility Tunnels as Preferential Pathways for Volatile Organic Compound Migration into Buildings: Risk Factors and Investigation Protocol, ESTCP Project ER-201505. [[media:2018b-McHugh-ER-201505_Conceptual_Model.pdf| Report.pdf]]</ref> |
| | + | *[[media:2018a-McHugh-ER-201505_Investigation_Protocol.pdf| Investigation protocol for sewer/utility tunnel VI]]<ref name= "McHugh2018a">McHugh, T. and Beckley, L., 2018. Sewers and Utility Tunnels as Preferential Pathways for Volatile Organic Compound Migration into Buildings: Risk Factors and Investigation Protocol. ESTCP Project ER-201505 [[media:2018a-McHugh-ER-201505_Investigation_Protocol.pdf| Report.pdf]]</ref> |
| | | | |
| − | ==Properties, Fate and Transport== | + | ==Introduction== |
| − | 1,4-Dioxane (14D) is a heterocyclic organic compound, and the most commonly encountered of the three dioxane isomers (1,2-, 1,3- and 1,4-Dioxane). Key physical and chemical properties of 14D are listed in Table 1. The heterocyclic, polar structure of 1,4-Dioxane causes it to be miscible with water and also unable to strongly sorb or partition to aquifer solids or engineered sorbents (i.e. activated carbon). While 14D has a moderate vapor pressure, the high aqueous solubility results in limited volatilization.
| + | A utility tunnel or utility corridor is a passage built underground or aboveground to carry utility lines such as electricity, water, and sewer pipes. Communication utilities like fiber optics, cable television, and telephone cables are also sometimes carried. They may also be referred to as a services tunnel, services trench, services vault, or cable vault. Utility tunnels are often installed in large military facilities as well as industrial plants and large institutions such as universities, hospitals, research labs, and other facilities managed in common. They are not generally installed in residential areas. A directly buried utility, such as an electrical or gas line, is not a utility tunnel. |
| − |
| + | |
| − | {| class="wikitable" style= "float:right; margin-left: 10px;" | + | Although not historically evaluated in a systematic manner, sanitary sewers and utility tunnels have been identified as important preferential transport pathways for VI at a growing number of sites<ref>McHugh, T., Beckley, L., Sullivan, T., Lutes, C., Truesdale, R., Uppencamp, R., Cosky, B., Zimmerman, J. and Schumacher, B., 2017a. Evidence of a sewer vapor transport pathway at the USEPA vapor intrusion research duplex. Science of the Total Environment, 598, pp.772-779. [https://doi.org/10.1016/j.scitotenv.2017.04.135 doi: 10.1016/j.scitotenv.2017.04.135]</ref><ref name= "McHugh2017b">McHugh, T., Loll, P. and Eklund, B., 2017. Recent advances in vapor intrusion site investigations. Journal of Environmental Management, 204, pp.783-792. doi: 10.1016/j.jenvman.2017.02.015 [[media:2017b-McHugh-Recent_advances_in_vapor_intrusion.pdf| Report.pdf]</ref>. They should, therefore, be considered during planning and implementation of VI assessment programs. Recognizing sewer/utility tunnel VI early in the site characterization process should help avoid wasted investigation efforts and delays in response actions where those are needed. |
| − | |+ Table 1. Properties of 1,4-Dioxane
| + | |
| | + | {| |
| | |- | | |- |
| − | ! Property
| + | | colspan="2" | Key terms and concepts: |
| − | ! Value
| |
| − | ! Reference
| |
| | |- | | |- |
| − | | Chemical Formula || C<sub>4</sub>H<sub>8</sub>O<sub>2</sub> || | + | | style="height:5px;" | |
| | |- | | |- |
| − | | Chemical Abstracts Service (CAS) Number || 123-91-1 || | + | | style="vertical-align:top; width:20%;" | Vapor intrusion || Migration of VOCs from any subsurface source into an overlying building. |
| | |- | | |- |
| − | | Appearance || Colorless Liquid || NIOSH, 2007<ref name= "NIOSH2007">Barsan, M.E., 2007. NIOSH pocket guide to chemical hazards. [[media:2007-NIOSH_Pocket_Guide_to_Chemical_Hazards.pdf| Report.pdf]]</ref> | + | | style="height:5px;" | |
| | |- | | |- |
| − | | Molecular Weight ||88.105 g/mol || NIST, 2018<ref>NIST, 2018. NIST Chemistry WebBook: Standard Reference Database 69. US Department of Commerce.</ref> | + | | style="vertical-align:top;" | Conventional, or standard, vapor intrusion |
| | + | | style="vertical-align:top;" | Migration of VOCs from a subsurface source into an overlying building by advection and/or diffusion through soil (i.e., not through a preferential pathway). These mechanisms for vapor entry into buildings can also be viewed as “soil gas intrusion.” Conventional VI refers to the conceptual model that has historically and most commonly been utilized to describe VOC flux from the subsurface into buildings. |
| | |- | | |- |
| − | | Water Solubility || Miscible || NIOSH, 2007<ref name= "NIOSH2007"/> | + | | style="height:5px;" | |
| | |- | | |- |
| − | | Specific Gravity || 1.03 || NIOSH, 2007<ref name= "NIOSH2007"/> | + | | style="vertical-align:top;" | Preferential pathway |
| | + | | style="vertical-align:top;" | A migration pathway from a subsurface source that supports higher VOC flux/discharge into a building compared to transport through bulk soil. This general term typically includes features such as elevator shafts and dry wells that can enhance vertical transport from a VOC source below the building into the building, and features such as sewers and utility tunnels that can enhance both lateral and vertical transport of VOCs. The term “sewer/utility tunnel vapor intrusion” or “sewer/utility tunnel VI” refers to VOC flux from the subsurface into buildings though this specific preferential pathway. |
| | |- | | |- |
| − | | Vapor Pressure at 25° C || 38.1 mm Hg || US EPA, 2017<ref name= "USEPA2017Z">US EPA, 2017. Technical Fact Sheet – 1,4-Dioxane. Publication number: EPA 505-F-17-001. [[media:2017-USEPA-_Technical_Fact_Sheet.pdf| Report.pdf]]</ref> | + | | style="height:5px;" | |
| | |- | | |- |
| − | | Henry’s Law Constant at 25° C || 4.80 X 10<sup>-6</sup> atm-m<sup>3</sup>/mol || US EPA, 2017<ref name= "USEPA2017Z"/> | + | | style="vertical-align:top;" | Sewer/utility tunnel vapor intrusion (sewer/utility tunnel VI) |
| | + | | style="vertical-align:top;" | A sewer or utility tunnel that supports higher VOC flux/discharge into a building compared to transport through bulk soil. The VOC flux is through the interior of the sewer line or tunnel. Sewer/utility tunnel vapor intrusion has also been referred to as “pipe VI”<ref name= "Guo2015">Guo, Y., Holton, C., Luo, H., Dahlen, P., Gorder, K., Dettenmaier, E. and Johnson, P.C., 2015. Identification of alternative vapor intrusion pathways using controlled pressure testing, soil gas monitoring, and screening model calculations. Environmental Science & Technology, 49(22), pp.13472-13482. [https://doi.org/10.1021/acs.est.5b03564 doi: 10.1021/acs.est.5b03564]</ref>. Sewers or utility tunnels can enhance VOC transport into a building from a VOC source that is laterally separated from the building (i.e., not located directly below the building). |
| | + | |} |
| | + | |
| | + | ==Conceptual Model== |
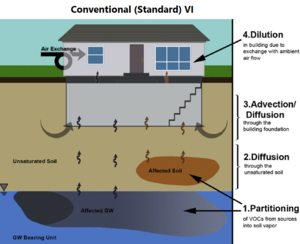
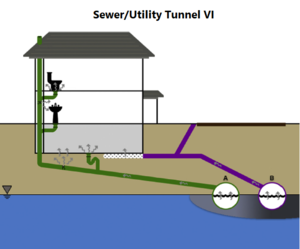
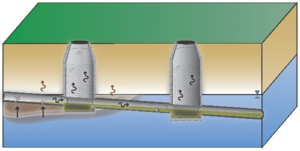
| | + | The standard VI and sewer/utility tunnel VI conceptual models are illustrated in Figure 1. One important difference is that, for sewer/utility tunnel VI, the building may be located outside the footprint of the subsurface contaminant source. In general, for standard VI, potentially affected buildings are situated over the source. |
| | + | [[File:Beckley1w2 Fig1a.png|thumb|left|Figure 1a. Conventional (Standard) Vapor Intrusion (from McHugh et al., 2017b<ref name= "McHugh2017b"/>)]] |
| | + | [[File:Beckley1w2 Fig1b.png|thumb|left|Figure 1b. Sewer/Utility Tunnel Vapor Intrusion (from McHugh et al., 2017b<ref name= "McHugh2017b"/>)]] |
| | + | |
| | + | {| class="wikitable" style="float:right; margin-left: 20px; text-align:center;" |
| | + | |+ Higher and Lower Risk Scenarios for Sewer/Utility Tunnel Vapor Intrusion |
| | |- | | |- |
| − | | Octanol-Water Partition Coefficient (log ''K<sub>ow</sub>'') || -0.27 || US EPA, 2017<ref name= "USEPA2017Z"/>
| + | ! Higher Risk Scenarios |
| | + | ! Lower Risk Scenario |
| | |- | | |- |
| − | | Organic Carbon Partition Coefficient (log ''K<sub>oc</sub>'') || 1.23 || Lyman et al., 1990<ref>Lyman, W.J., W.F. Reehl , D.H. Rosenblatt, and N.P.E. Vermeulen, 1990. Handbook of Chemical Property Estimation Methods. American Chemical Society, 110(2), 61-61. [https://doi.org/10.1002/recl.19911100212 doi: 10.1002/recl.19911100212]</ref> | + | | style="padding: 35px;" | [[File:Beckley1w2_Fig2a.png | thumbnail | Figure 2a. Sewer Intersects Contaminated Groundwater]] |
| | + | | style="padding: 35px;" | [[File:Beckley1w2_Fig2d.png | thumbnail | Figure 2d. Sewer in Vadose Zone above Plume]] |
| | + | |- |
| | + | | style="padding: 35px;" | [[File:Beckley1w2_Fig2b.png | thumbnail | Figure 2b. Discharge of Groundwater to Sewer Line]] || |
| | + | |- |
| | + | | style="padding: 35px;" | [[File:Beckley1w2_Fig2c.png | thumbnail | Figure 2c. Sewer Intersects NAPL/Vadose Zone Source]] |
| | + | | style="padding: 35px;" | [[File:Beckley1w2_Fig2_legend.png | 200px]] |
| | |} | | |} |
| | | | |
| − | Biodegradation of 14D is limited in many environments including surface water, groundwater and wastewater treatment plants (see [Biodegradation – 1,4-Dioxane]). Currently, there is little evidence that 14D biodegrades under anaerobic conditions<ref>Shen, W., Chen, H. and Pan, S., 2008. Anaerobic biodegradation of 1, 4-dioxane by sludge enriched with iron-reducing microorganisms. Bioresource technology, 99(7), pp.2483-2487. [https://doi.org/10.1016/j.biortech.2007.04.054 doi: 10.1016/j.biortech.2007.04.054]</ref><ref name= "Zhang2017">Zhang, S., Gedalanga, P.B. and Mahendra, S., 2017. Advances in bioremediation of 1, 4-dioxane-contaminated waters. Journal of environmental management, 204, pp.765-774. [https://doi.org/10.1016/j.jenvman.2017.05.033 doi: 10.1016/j.jenvman.2017.05.033]</ref>. In contrast, under aerobic conditions, several bacteria and fungi<ref>Skinner, K., Cuiffetti, L. and Hyman, M., 2009. Metabolism and cometabolism of cyclic ethers by a filamentous fungus, a Graphium sp. Appl. Environ. Microbiol., 75(17), pp.5514-5522. [https://doi.org/10.1128/aem.00078-09 doi:10.1128/AEM.00078-09]</ref> can grow on 14D as a sole source of carbon and energy, including well-characterized strains such as ''Pseudonocardia dioxanivorans'' CB1190<ref name= "Parales1994">Parales, R.E., Adamus, J.E., White, N. and May, H.D., 1994. Degradation of 1, 4-dioxane by an actinomycete in pure culture. Applied and Environmental Microbiology, 60(12), pp.4527-4530. [[media:1994-Parales-Degradation_of_1%2C4-Dioxane_by_an_Actinomycete_in_Pure_Culture.pdf| Report.pdf]]</ref><ref name= "Mahendra2006">Mahendra, S. and Alvarez-Cohen, L., 2006. Kinetics of 1, 4-dioxane biodegradation by monooxygenase-expressing bacteria. Environmental Science & Technology, 40(17), pp.5435-5442. [https://doi.org/10.1021/es060714v [https://doi.org/10.1021/es060714v doi: 10.1021/es060714v]</ref>) and other closely related actinomycetes<ref name= "Zhang2017"/><ref>Yamamoto, N., Saito, Y., Inoue, D., Sei, K. and Ike, M., 2018. Characterization of newly isolated Pseudonocardia sp. N23 with high 1, 4-dioxane-degrading ability. Journal of bioscience and bioengineering, 125(5), pp.552-558. [https://doi.org/10.1016/j.jbiosc.2017.12.005 doi: 10.1016/j.jbiosc.2017.12.005]</ref><ref>Inoue, D., Tsunoda, T., Sawada, K., Yamamoto, N., Saito, Y., Sei, K. and Ike, M., 2016. 1, 4-Dioxane degradation potential of members of the genera Pseudonocardia and Rhodococcus. Biodegradation, 27(4-6), pp.277-286. [https://doi.org/10.1007/s10532-016-9772-7 doi: 10.1007/s10532-016-9772-7]</ref><ref>Kim, Y.M., Jeon, J.R., Murugesan, K., Kim, E.J. and Chang, Y.S., 2009. Biodegradation of 1, 4-dioxane and transformation of related cyclic compounds by a newly isolated Mycobacterium sp. PH-06. Biodegradation, 20(4), p.511. [https://doi.org/10.1007/s10532-008-9240-0 doi: 10.1007/s10532-008-9240-0]</ref>. However, growth of these organisms on 14D is slow<ref>Barajas-Rodriguez, F.J. and Freedman, D.L., 2018. Aerobic biodegradation kinetics for 1, 4-dioxane under metabolic and cometabolic conditions. Journal of hazardous materials, 350, pp.180-188. [https://doi.org/10.1016/j.jhazmat.2018.02.030 doi: 10.1016/j.jhazmat.2018.02.030]</ref>, and as a consequence, 14D-metabolizing microorganisms are generally not effective in degrading 14D at the lower concentrations (≤100 μg/L) commonly observed in surface water and groundwater <ref name= "Adamson2014">Adamson, D.T., Mahendra, S., Walker Jr, K.L., Rauch, S.R., Sengupta, S. and Newell, C.J., 2014. A multisite survey to identify the scale of the 1, 4-dioxane problem at contaminated groundwater sites. Environmental Science & Technology Letters, 1(5), pp.254-258. [https://doi.org/10.1021/ez500092u doi: 10.1021/ez500092u]</ref><ref name= "Knappe2016">Knappe, D., Lopez-Velandia, C., Hopkins, Z. and Sun, M., 2016. Occurrence of 1, 4-Dioxane in the Cape Fear River Watershed and Effectiveness of Water Treatment Options for 1, 4-Dioxane Control. NC Water Resources Research Institute of The University of North Carolina, Report No. 478. [[media:2016-Knappe-Occurrence_of_1%2C4-dioxane_in_the_Cape_Fear_River.pdf| Report.pdf]]</ref>.
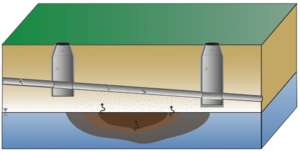
| + | Not all sites are equally at risk for sewer/utility tunnel VI. In general, higher risk sites are those where contaminated groundwater or soil gas has infiltrated into the underground utility line. This occurs most commonly at sites with sewers or utility tunnels that intersect shallow impacted water tables, sites with discharge of contaminated groundwater into sanitary sewer lines, or sites with sewer lines or utility tunnels that intersect strong contaminant sources (e.g., nonaqueous phase liquid (NAPL)) in the unsaturated zone (Figure 2). |
| | | | |
| − | While 14D is not commonly used as a microbial growth substrate at ambient concentrations, there are a wide variety of microorganisms that can [https://enviro.wiki/index.php?title=Biodegradation_-_Cometabolic cometabolically biodegrade] 14D when grown on a different (primary) substrate. During cometabolism, the primary substrate supports production of active biomass and induces the appropriate enzymes that can then fortuitously biodegrade 14D. Primary substrates that have been shown to support cometabolic biodegradation of 14D include tetrahydrofuran (THF), toluene, methane, ethane, propane, and isobutane<ref name= "Mahendra2006"/><ref>Vainberg, S., McClay, K., Masuda, H., Root, D., Condee, C., Zylstra, G.J. and Steffan, R.J., 2006. Biodegradation of ether pollutants by Pseudonocardia sp. strain ENV478. Appl. Environ. Microbiol., 72(8), pp.5218-5224. [https://doi.org/10.1128/aem.00160-06 doi: 10.1128/AEM.00160-06]</ref><ref>Hatzinger, P.B., Banerjee, R., Rezes, R., Streger, S.H., McClay, K. and Schaefer, C.E., 2017. Potential for cometabolic biodegradation of 1, 4-dioxane in aquifers with methane or ethane as primary substrates. Biodegradation, 28(5-6), pp.453-468. [https://doi.org/10.1007/s10532-017-9808-7 doi: 0.1007/s10532-017-9808-7]</ref><ref>Bennett, P., Hyman, M., Smith, C., El Mugammar, H., Chu, M.Y., Nickelsen, M. and Aravena, R., 2018. Enrichment with carbon-13 and deuterium during monooxygenase-mediated biodegradation of 1, 4-dioxane. Environmental Science & Technology Letters, 5(3), pp.148-153. [https://doi.org/10.1021/acs.estlett.7b00565 doi: 10.1021/acs.estlett.7b00565]</ref><ref>Deng, D., Li, F., Wu, C. and Li, M., 2018. Synchronic Biotransformation of 1, 4-Dioxane and 1, 1-Dichloroethylene by a Gram-Negative Propanotroph Azoarcus sp. DD4. Environmental Science & Technology Letters, 5(8), pp.526-532. [https://doi.org/10.1021/acs.estlett.8b00312 doi: 10.1021/acs.estlett.8b00312]</ref>. Recent field studies have demonstrated that propane-stimulated cometabolic biodegradation can reduce 14D to below 2-3 µg/L under appropriate conditions<ref name= "Lippincott2015">Lippincott, D., Streger, S.H., Schaefer, C.E., Hinkle, J., Stormo, J. and Steffan, R.J., 2015. Bioaugmentation and propane biosparging for in situ biodegradation of 1, 4‐dioxane. Groundwater Monitoring & Remediation, 35(2), pp.81-92. [https://doi.org/10.1111/gwmr.12093 doi: 10.1111/gwmr.12093]</ref><ref name= "Chu2018">Chu, M.Y.J., Bennett, P.J., Dolan, M.E., Hyman, M.R., Peacock, A.D., Bodour, A., Anderson, R.H., Mackay, D.M. and Goltz, M.N., 2018. Concurrent Treatment of 1, 4‐Dioxane and Chlorinated Aliphatics in a Groundwater Recirculation System Via Aerobic Cometabolism. Groundwater Monitoring & Remediation, 38(3), pp.53-64. [https://doi.org/10.1111/gwmr.12293 doi: 10.1111/gwmr.12293]</ref>.
| + | Elevated VOC concentrations in sewer vapor can be found at both higher and lower risk sites. At higher risk sites, these elevated VOC concentrations can be present over a larger area, extending beyond the footprint of the subsurface contamination. Standard VI investigations typically focus on areas above this footprint. To factor in sewer/utility tunnel VI, it is important to identify higher risk sites early in the VI investigation process so that potential impacts outside the footprint are not overlooked. |
| | | | |
| − | As a result of the low sorption, low volatilization and low biodegradation, attenuation of 14D is often limited in groundwater<ref>Nyer, E., Boettcher, G. and Morello, B., 1991. Using the properties of organic compounds to help design a treatment system. Groundwater Monitoring & Remediation, 11(4), pp.81-86. [https://doi.org/10.1111/j.1745-6592.1991.tb00395.x doi: 10.1111/j.1745-6592.1991.tb00395.x]</ref><ref>Jackson, R.E. and Dwarakanath, V., 1999. Chlorinated decreasing solvents: physical‐chemical properties affecting aquifer contamination and remediation. Groundwater Monitoring & Remediation, 19(4), pp.102-110. [https://doi.org/10.1111/j.1745-6592.1999.tb00246.x doi: 10.1111/j.1745-6592.1999.tb00246.x]</ref>, surface water<ref name= "Knappe2016"/> and traditional potable and wastewater treatment systems<ref name = "Stepien2014">Stepien, D.K., Diehl, P., Helm, J., Thoms, A. and Püttmann, W., 2014. Fate of 1, 4-dioxane in the aquatic environment: From sewage to drinking water. Water Research, 48, pp.406-419. [https://doi.org/10.1016/j.watres.2013.09.057 doi: 10.1016/j.watres.2013.09.057]</ref>. In groundwater, the limited sorption of 14D results in relatively low concentrations in the source area and large dilute plumes<ref name= "Adamson2014"/>. Under these conditions, much of the 14D mass will be present in the downgradient plume and aggressive treatment of the source area is generally less effective in reducing 14D migration.
| + | ==Investigation Approaches== |
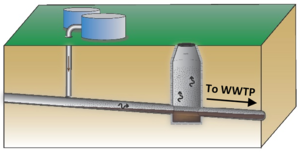
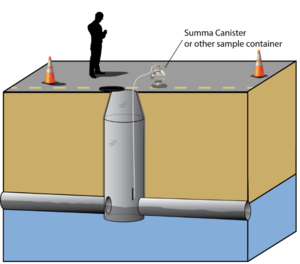
| | + | Basic screening for sewer/utility tunnel VI can be done at any site by determining if the site has higher or lower risk for this pathway. If the site has higher risk, initial testing can be done by sampling vapor from manholes (Figure 3; Also add link to video) above the core of the groundwater plume to get an understanding of the level of VOCs that may be present. |
| | + | [[File:Beckley1w2 Fig3.png|thumb|left|Figure 3. Vapor Sample Collection from Sewer]] |
| | | | |
| − | ==Toxicity and Regulatory Standards (updated 2018)==
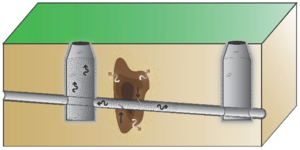
| + | Follow-up testing may be needed depending on the initial results. Vapor intrusion guidance documents generally do not provide screening values for sewer vapor samples. However, results from ESTCP Project ER-201505 show that sewer to indoor air attenuation is similar in magnitude to sub-slab to indoor air attenuation. This suggests that sub-slab screening values can also be used to evaluate sewer vapor results<ref name= "McHugh2018a"/>. VOC vapors can migrate from sewers into buildings through a variety of different entry mechanisms that are difficult to predict (Figure 4). As a result, if sewer vapor concentrations exceed screening levels, then indoor air testing may be needed to determine the presence or absence of VI. |
| − | The U.S. Department of Health and Human Services<ref>ATSDR, 2012. Toxicological profile for 1,4-dioxane. Agency for Toxic Substances and Disease Registry [[media:2012-ASTDR._Toxicological_profile_for_1%2C4-dioxane.pdf| Report.pdf]]</ref> classifies 14D as a reasonably anticipated human carcinogen and the International Agency for Research on Cancer<ref name= "IARC1999">IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, International Agency for Research on Cancer and World Health Organization, 1999. Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide. IARC.</ref><ref>Stickney, J.A., Sager, S.L., Clarkson, J.R., Smith, L.A., Locey, B.J., Bock, M.J., Hartung, R. and Olp, S.F., 2003. An updated evaluation of the carcinogenic potential of 1, 4-dioxane. Regulatory Toxicology and Pharmacology, 38(2), pp.183-195. [https://doi.org/10.1016/S0273-2300(03)00090-4 doi: 10.1016/S0273-2300(03)00090-4]</ref> regards 14D as possibly carcinogenic to humans (Group 2B). These classifications are primarily based on research on mice, rats and guinea pigs<ref name= "IARC1999"/>. One human epidemiological study reported no increased deaths from cancer in workers exposed to 14D<ref>Buffler, P.A., Wood, S.M., Suarez, L. and Kilian, D.J., 1978. Mortality follow-up of workers exposed to 1, 4-dioxane. Journal of occupational medicine.: official publication of the Industrial Medical Association, 20(4), pp.255-259.</ref>.
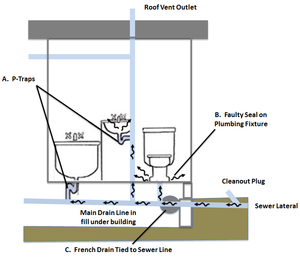
| + | [[File:Beckley1w2 Fig4.png|thumb|Figure 4. Potential Entry Points into Buildings. VOCs can move from sewers and utility tunnels into buildings through a variety of features, for example: A. Dry p-traps; B. Faulty seal on plumbing fixture<ref>Pennell, K.G., Scammell, M.K., McClean, M.D., Ames, J., Weldon, B., Friguglietti, L., Suuberg, E.M., Shen, R., Indeglia, P.A. and Heiger‐Bernays, W.J., 2013. Sewer gas: an indoor air source of PCE to consider during vapor intrusion investigations. Groundwater Monitoring & Remediation, 33(3), pp.119-126. [https://doi.org/10.1111/gwmr.12021 doi: 10.1111/gwmr.12021]</ref>; and C. French drain tied to sewer line<ref name= "Guo2015"/>. Utility tunnels can vent directly into buildings.]] |
| | | | |
| − | There is currently no maximum contaminant level (MCL) for 14D. However, tap water screening (0.46 µg/L) and drinking water equivalent levels (1 mg/L)<ref>U.S. Environmental Protection Agency (USEPA), 2018(a). Edition of the Drinking Water Standards and Health Advisories. EPA 822-F-18-001 [[media:2018-USEPA-Edition_of_the_Drinking_Water_Standards_and_Health_Advisories.pdf| Report.pdf]]</ref> have been established. For soil contamination, the US EPA has calculated a residential soil screening level (SSL) of 5.3 milligrams per kilogram (mg/kg), an industrial SSL of 24 mg/kg and leach-based SSL of 9.4 x 10<sup>-5</sup> mg/kg <ref>U.S. Environmental Protection Agency (USEPA), 2018(b). Regional Screening Level (RSL) Summary Tables. </ref>. Many states have established drinking water and groundwater standards, which range from 0.25 (New Hampshire) to 9.1 µg/L (Texas)<ref>Suthersan, S., Quinnan, J., Horst, J., Ross, I., Kalve, E., Bell, C. and Pancras, T., 2016. Making strides in the management of “emerging contaminants”. Groundwater Monitoring & Remediation, 36(1), pp.15-25. [https://doi.org/10.1111/gwmr.12143 doi: 10.1111/gwmr.12143]</ref>.
| + | At lower risk sites, a specific, early focus on sewers or utility tunnels is generally not needed. A standard VI investigation that includes indoor air testing can be conducted with evaluation of sewers/utility tunnels only if indicated by the building-specific investigation results (e.g., if elevated indoor air concentrations in one section of the building suggest a specific vapor entry point). |
| | | | |
| − | ==History of Use and Release to Environment==
| + | Many indoor air testing techniques utilized to assess conventional vapor intrusion are also applicable to buildings potentially impacted by sewer/utility tunnel VI. Techniques that focus on VOC source identification (e.g., indoor VOC sources vs. vapor entry points) include on-site analysis, compound-specific isotope analysis, and building pressure cycling. |
| − | The most common use of 14D has been as a solvent stabilizer for 1,1,1-trichloroethane (TCA) utilized in metal degreasing operations. The addition of 14D to degreasing solvents such as TCA inhibits the formation of metal salts, which can result in solvent breakdown<ref name= "Mohr2010"/>. Approximately 90% of 14D produced in the 1980s was used as a stabilizer for TCA<ref name= "Mohr2010"/>. Following the [https://en.wikipedia.org/wiki/Montreal_Protocol phase-out of ozone depleting chemicals] including TCA, the production and use of 14D has declined. Beyond its use as a solvent stabilizer, 14D has also been used in textile, paint and ink, cellulose acetate membrane and adhesive manufacturing operations<ref name= "Mohr2010"/>.
| |
| | | | |
| − | 14D is an un-intended byproduct of some organic chemical synthesis processes<ref name= "Mohr2010"/>. 14D can be produced during manufacture of alcohol ethoxylates which are used in a wide variety of consumer products including soaps and detergents, cosmetics and food. In 2001, ethoxylated raw materials were found to contain up to 1.4% 14D<ref>Black, R.E., Hurley, F.J. and Havery, D.C., 2001. Occurrence of 1, 4-dioxane in cosmetic raw materials and finished cosmetic products. Journal of AOAC international, 84(3), pp.666-670. [[media:2001-Black-Occurrence_of_1%2C4-dioxane_in_cosmetic_raw_materials....pdf| Report.pdf]]</ref>. Once this issue was recognized, measures were put into place to reduce 14D levels. 14D levels in the final product can be reduced by controlling the production process<ref>Ortega, J.A.T., 2012. Sulfonation/sulfation processing technology for anionic surfactant manufacture. In Advances in Chemical Engineering. IntechOpen. [[media:2012-Ortega-Sulfonation_sulfation.pdf| Report.pdf]]</ref> and by treatment of finished products by several approaches including vacuum stripping, steam stripping, and drying<ref>Sachdeva, Y.P. and Gabriel, R.L., Pharm-Eco Laboratories Inc, 1997. Apparatus for decontaminating a liquid surfactant of dioxane. U.S. Patent 5,643,408. [[media:1997-Sachdeva-Apparatus_for_decontaminating_a_liquid_surfractant.pdf| Report.pdf]]</ref>. 14D is produced as a byproduct during production of polyethylene terephthalate (PET), polyester and strong surfactants, as well as many other products<ref>Ellis, R.A. and Thomas, J.S., Wellman Inc, 1998. Destroying 1, 4-dioxane in byproduct streams formed during polyester synthesis. U.S. Patent 5,817,910. [[media:1998-Ellis-Destroying_1%2C4-dioxane_in_byproduct_streams_formed_during_polyester.pdf| report.pdf]]</ref>. Treated industrial wastewater containing 14D may be released to surface water or groundwater<ref name= "Knappe2016"/><ref name= "Grady1997">Grady, C.P.L., Sock, S.M. and Cowan, R.M., 1997. Biotreatability Kinetics: A Critical Component in the Scale-up of Wastewater Treatment Systems. Environmental Science Research, 54, pp.307-322. [https://doi.org/10.1007/978-1-4615-5395-3_28 doi: 10.1007/978-1-4615-5395-3_28]</ref><ref name= "Zenker 2000">Zenker, M.J., Borden, R.C. and Barlaz, M.A., 2000. Mineralization of 1, 4-dioxane in the presence of a structural analog. Biodegradation, 11(4), pp.239-246. [https://doi.org/10.1023/A:1011156924700 doi: 10.1023/A:1011156924700]</ref>
| + | ==Mitigation== |
| | + | If sewer/utility tunnel VI is identified, mitigation approaches include preventing contaminants from entering the sewer or utility (e.g., by rerouting or lining the sewer), ventilating the line, or correcting plumbing defects. Examples are provided in Table 1. |
| | | | |
| − | 14D has been detected in air, potable water, wastewater and groundwater. 14D was detected in ambient air in several studies<ref>Harkov, R., Katz, R., Bozzelli, J. and Kebbekus, B., 1981. Toxic and carcinogenic air pollutants in New Jersey volatile organic substances. Proceedings of the International Technical Conference of Toxic Air Contaminants, p.245. Pittsburgh, PA: Air Pollution Control Association</ref><ref>Shah, J.J. and Singh, H.B., 1988. Distribution of volatile organic chemicals in outdoor and indoor air: A national VOCs data base. Environmental Science & Technology, 22(12), pp.1381-1388. [https://pubs.acs.org/doi/abs/10.1021/es00177a001 doi: 10.1021/es00177a001]</ref><ref>Keel, L. and A. Franzmann, 2000. Downtown Bellingham air toxics screening project, 1995-1999 staff report. Northwest Air Pollution Authority (NWAPA). [[media:2000-Keel-Downtown_Bellingham_Air_Toxics_Screening_Project.pdf| Report.pdf]]</ref>). In a survey of 4,864 public drinking water systems, 14D was detected in 21% of the systems and exceeded the health-based reference concentration of 0.35 g/L in 6.9%<ref name= "Adamson2017">Adamson, D.T., Piña, E.A., Cartwright, A.E., Rauch, S.R., Anderson, R.H., Mohr, T. and Connor, J.A., 2017. 1, 4-Dioxane drinking water occurrence data from the third unregulated contaminant monitoring rule. Science of the Total Environment, 596, pp.236-245. [https://doi.org/10.1016/j.scitotenv.2017.04.085 doi: 10.1016/j.scitotenv.2017.04.085]</ref>. Municipal wastewater<ref>Abe, A., 1999. Distribution of 1, 4-dioxane in relation to possible sources in the water environment. Science of the Total Environment, 227(1), pp.41-47. [https://doi.org/10.1016/S0048-9697(99)00003-0 doi: 10.1016/S0048-9697(99)00003-0]</ref><ref>Westerhoff, P., Yoon, Y., Snyder, S. and Wert, E., 2005. Fate of endocrine-disruptor, pharmaceutical, and personal care product chemicals during simulated drinking water treatment processes. Environmental Science & Technology, 39(17), pp.6649-6663. [https://doi.org/10.1021/es0484799 doi: 10.1021/es0484799]</ref> and landfill leachates<ref>DeWalle, F.B. and Chian, E.S., 1981. Detection of trace organics in well water near a solid waste landfill. Journal‐American Water Works Association, 73(4), pp.206-211. [https://doi.org/10.1002/j.1551-8833.1981.tb04681.x doi: 10.1002/j.1551-8833.1981.tb04681.x]</ref><ref>Fujiwara, T., Tamada, T., Kurata, Y., Ono, Y., Kose, T., Ono, Y., Nishimura, F. and Ohtoshi, K., 2008. Investigation of 1, 4-dioxane originating from incineration residues produced by incineration of municipal solid waste. Chemosphere, 71(5), pp.894-901. [https://doi.org/10.1016/j.chemosphere.2007.11.011 doi: 10.1016/j.chemosphere.2007.11.011]</ref> also frequently contain 14D.
| + | {|class="wikitable" |
| − | | + | |+ Table 1. Examples of Sewer Mitigation Methods Used to Control Vapor Intrusion |
| − | In groundwater, 14D is frequently observed as a co-contaminant with TCA<ref name= "Adamson2014"/><ref name= "Anderson2012">Anderson, R.H., Anderson, J.K. and Bower, P.A., 2012. Co‐occurrence of 1, 4‐dioxane with trichloroethylene in chlorinated solvent groundwater plumes at US Air Force installations: Fact or fiction. Integrated Environmental Assessment and Management, 8(4), pp.731-737. [https://doi.org/10.1002/ieam.1306 doi: 10.1002/ieam.1306]</ref>. As 14D was often utilized as an additive to degreasing solvents<ref name= "Mohr2010"/>, its presence is positively correlated with other chlorinated organics such as TCA, trichloroethene (TCE), and 1,1-dichloroethene (1,1-DCE)<ref name= "Adamson2017"/> TCA and/or TCE were observed in 94% of the monitoring wells with detectable 14D<ref name= "Anderson2012"/>. In a review of groundwater results from 194 sites with detectable 14D, the median value of the range of maximal historical dioxane concentrations was 365 μg/L (10th percentile, 9 μg/L; 90th percentile, 13460 μg/L)<ref name= "Adamson2014"/>.
| + | |- |
| − | | + | ! Mitigation Method |
| − | The common presence of 14D in potable water supplies is a concern because 14D removal is low in many unit processes commonly employed in publicly owned treatment works (POTW) due to the high solubility, low volatility, and relative resistance to biodegradation (see section on physical/chemical treatment). 14D removal was not observed in several physical/chemical water treatment processes including coagulation, oxidation and air stripping processes<ref name= "McGuire1978">McGuire, M.J., Suffet, I.H. and Radziul, J.V., 1978. Assessment of unit processes for the removal of trace organic compounds from drinking water. Journal‐American Water Works Association, 70(10), pp.565-572. [https://doi.org/10.1002/j.1551-8833.1978.tb04244.x doi: 10.1002/j.1551-8833.1978.tb04244.x ]</ref>. Activated carbon adsorption is somewhat more successful, with reported removal efficiencies ranging from 50 to 67 percent<ref name= "McGuire1978"/><ref>Johns, M.M., Marshall, W.E. and Toles, C.A., 1998. Agricultural by‐products as granular activated carbons for adsorbing dissolved metals and organics. Journal of Chemical Technology & Biotechnology: International Research in Process, Environmental and Clean Technology, 71(2), pp.131-140. [https://doi.org/10.1002/(SICI)1097-4660(199802)71:2<131::AID-JCTB821>3.0.CO;2-K doi: 10.1002/(SICI)1097-4660(199802)71:2<131::AID-JCTB821>3.0.CO;2-K]</ref><ref name = "Stepien2014"/> reported that 14D removal was not observed in four domestic wastewater treatment plants in Germany. In a study of North Carolina water treatment facilities, 14D removal was not observed at two of the facilities, but was reduced by 65% at a third which employed ozonation of raw and settled water<ref name= "Knappe2016"/>.
| + | ! Reference |
| − | | + | |- |
| − | ==Above Ground Treatment==
| + | | Depressurization of sewer line || Nielsen et al., 2014<ref>Nielsen, K.B., Hivdberg, B. and Hyldegaard, W., 2014. Vinyl chloride in the indoor air solved by depressurization of the sewer. In Battelle Ninth International Conference on Remediation of Chlorinated and Recalcitrant Compounds Monterey, CA.</ref> |
| − | Air stripping is generally not effective for removing 14D from contaminated water due its low Henry’s Law constant. While 14D can be removed from water by sorption to granular activated carbon (GAC), sorption capacities are low resulting in high capital and operating costs<ref name= "Woodard2014">Woodard, S., Mohr, T. and Nickelsen, M.G., 2014. Synthetic Media: A Promising New Treatment Technology for 1, 4‐Dioxane. Remediation Journal, 24(4), pp.27-40. [https://doi.org/10.1002/rem.21402 doi:10.1002/rem.21402]</ref>. In contrast, polymeric sorbents (e.g. Ambersorb<sup>TM</sup> 560) are reported to have 5 to 10 times the sorption capacity of GAC, making sorption a more attractive alternative, though with additional operational requirements of adsorbent regeneration and proper disposal of spent waste<ref name= "Woodard2014"/>. While 14D can be degraded by high energy UV radiation in high purity water<ref>Hentz, R.R. and Parrish, C.F., 1971. Photolysis of gaseous 1, 4-dioxane at 1470 Ang. The Journal of Physical Chemistry, 75(25), pp.3899-3901. [https://doi.org/10.1021/j100694a023 doi: 10.1021/j100694a023]</ref>, direct ultraviolet photolysis is not economically viable due to operational challenges and high costs<ref name= "Mohr2010"/>.
| + | |- |
| − | | + | | Replacement of collapsed portion of sanitary sewer line and installation of an interior liner to prevent infiltration of LNAPL || Macklin et al., 2014<ref>Macklin, Y., Welfare, W., Kowalczyk, G., Mitchem, L., Modi, A., Craswell, A., Brown, M. and Lighton, L., 2014. Sewers, culverts and other underground pipes–an under recognised pathway for chemical exposures in acute incidents: case series. Chemical Hazards and Poisons Report, p.15. [[media:2014-Chemical_Hazards_and_Poisons_Rpt.pdf| Report.pdf]]</ref> |
| − | Advanced oxidation processes (AOPs) using combinations of ozone (O<sub>3</sub>), hydrogen peroxide (H<sub>2</sub>O<sub>2</sub>) and ultraviolet (UV) radiation produce highly reactive hydroxyl radicals (•OH) which are effective in destroying 14D. In the peroxone process (O<sub>3,</sub> + H<sub>2,</sub>O<sub>2</sub>), O<sub>3</sub> reacts with H<sub>2</sub>O<sub>2</sub> giving rise to hydroxyl radicals<ref>Fleming, E.C., Zappi, M.E., Toro, E., Hernandez, R., Myers, K., Kodukula, P. and Gilbertson, R., 1997. Laboratory assessment of advanced oxidation processes for treatment of explosives and chlorinated solvents in groundwater from the former Nebraska Ordnance Plant. Technical Report SERDP-97-3 [[media:1997-Fleming-Laboratory_assessment_of_advanced_oxidation_process.pdf| Report.pdf]]</ref>. In a variation of this approach ([http://aptwater.com/hipox/technology/overview-advanced-oxidation-process-for-water-treatment/ HiPOx™ process]), H<sub>2</sub>O<sub>2</sub> is added first followed by high pressure O<sub>3</sub><ref>Bowman, R.H., Lahey, T. and Herlihy, P., Applied Process Tech Inc, 2007. System and method for remediating contaminated soil and groundwater in situ. U.S. Patent 7,264,419. [[media:2007-Bowman-System_and_method_fo_remediating_contaminated_soil_and_groundwater.pdf| Report .pdf]]</ref>. Hydroxyl radicals are produced in UV + H<sub>2</sub>O<sub>2</sub> systems by direct photolysis of H<sub>2</sub>O<sub>2</sub> caused by absorption of energy from an ultraviolet lamp<ref>Omura, K. and Matsuura, T., 1968. Photo-induced reactions—IX: The hydroxylation of phenols by the photo-decomposition of hydrogen peroxide in aqueous media. Tetrahedron, 24(8), pp.3475-3487. [https://doi.org/10.1016/S0040-4020(01)92645-6 doi: 10.1016/S0040-4020(01)92645-6]</ref><ref>Stefan, M.I. and Bolton, J.R., 1998. Mechanism of the degradation of 1, 4-dioxane in dilute aqueous solution using the UV/hydrogen peroxide process. Environmental Science & Technology, 32(11), pp.1588-1595. [https://doi.org/10.1021/es970633m doi: 10.1021/es970633m]</ref>.
| + | |- |
| − | | + | | Sewer line relocation to avoid intersecting contaminated groundwater plume || ERM, 2017<ref name= "ERM2017">ERM, 2017. Vapor Intrusion Evaluation Activities Summary Report - February to December 2016, Indianapolis, Indiana. [[media:2017-ERM-Vapor_Intrusion_Eval_Activities_Summary.pdf| Report.pdf]]</ref> |
| − | AOPs are commonly employed for 14D treatment because of their high removal efficiencies with low contact times<ref name= "Mohr2010"/><ref name= "Ikehata">Ikehata, K., Jodeiri Naghashkar, N. and Gamal El-Din, M., 2006. Degradation of aqueous pharmaceuticals by ozonation and advanced oxidation processes: a review. Ozone: Science and Engineering, 28(6), pp.353-414. [https://doi.org/10.1080/01919510600985937 doi: 10.1080/01919510600985937]</ref>. The degradation mechanisms and kinetics of these processes are well understood, with multiple systems in current use<ref name= "Mohr2010"/>. All of these systems are effective in destroying 14D, but have some limitations including the potential for bromate formation (when Br is present), reduced efficiency in the presence of free radical scavengers (e.g. HCO<sub>3</sub>) and high capital and operating costs associated with energy and chemical consumption<ref name= "Mohr2010"/><ref>Woodward, C., Shulmeister, J., Larsen, J., Jacobsen, G.E. and Zawadzki, A., 2014. The hydrological legacy of deforestation on global wetlands. Science, 346(6211), pp.844-847. [https://doi.org/10.1126/science.1260510 doi: 10.1126/science.1260510]</ref>.
| + | |- |
| − | | + | | Various options including: |
| − | Biological wastewater treatment has not been widely applied for 14D removal. While high concentrations of 14D will support growth of 14D degraders<ref>Sock, S.M., 1993. A comprehensive evaluation of biodegradation as a treatment alternative for the removal of 1, 4-dioxane (Doctoral dissertation, Clemson University)</ref> growth rates on 14D are slow<ref name= "Grady1997"/><ref name= "Parales1994"/><ref>Roy, D., Anagnostu, G. and Chaphalkar, P., 1994. Biodegradation of dioxane and diglyme in industrial waste. Journal of Environmental Science & Health Part A, 29(1), pp.129-147. [https://doi.org/10.1080/10934529409376026 doi: 10.1080/10934529409376026]</ref><ref>Bernhardt, D. and Diekmann, H., 1991. Degradation of dioxane, tetrahydrofuran and other cyclic ethers by an environmental Rhodococcus strain. Applied Microbiology and Biotechnology, 36(1), pp.120-123. [https://doi.org/10.1007/bf00164711 doi: 10.1007/BF00164711]</ref> and the low 14D concentrations in many systems are not sufficient to maintain an adequate level of 14D degraders for effective treatment. To overcome this problem, 14D degrading biomass was supported by addition of tetrahydrofuran (THF) to a continuously fed trickling filter for over 1 year. The system was effective in reducing 14D concentrations by 93–97% while influent 14D concentrations were varied from 200 to 1200 µg/L<ref>Zenker, M.J., Borden, R.C. and Barlaz, M.A., 2003. Occurrence and treatment of 1, 4-dioxane in aqueous environments. Environmental Engineering Science, 20(5), pp.423-432. [https://doi.org/10.1089/109287503768335913 doi: 10.1089/109287503768335913]</ref>. This basic approach was applied to full-scale treatment of leachate from the Lowry Landfill Superfund site where both THF and 14D were present as co-contaminants<ref>Shangraw, T. and Plaehn, W. 2012. Full-scale treatment of 1,4-dioxane using a bioreactor. Federal Remediation Technologies Roundtable Meeting. [[media:2012-Shangraw-Full-scale_Treatment_of_1%2C4-dioxane.pdf | Report.pdf]]</ref>.
| + | * Repairing or lining sewer line to prevent infiltration of liquids or vapors |
| − | | + | * Sealing or repairing leaky/damaged water traps inside building |
| − | ==''In-Situ'' Treatment==
| + | * Passive ventilation of manholes |
| − | A variety of methods have been attempted for ''in situ'' treatment of 14D impacted soil and groundwater including [https://enviro.wiki/index.php?title=Chemical_Oxidation_(In_Situ_-_ISCO) in situ chemical oxidation (ISCO)], thermally enhanced soil vapor extraction, bioremediation, phytoremediation, and [https://enviro.wiki/index.php?title=Monitored_Natural_Attenuation_(MNA) monitored natural attenuation (MNA)].
| + | * Depressurization of sewer system |
| − | | + | | Nielsen and Hivdberg, 2017<ref name= "Nielsen2017">Nielsen, K.B. and Hvidberg, B., 2017. Remediation techniques for mitigating vapor intrusion from sewer systems to indoor air. Remediation Journal, 27(3), pp.67-73. [https://doi.org/10.1002/rem.21520 doi: 10.1002/rem.21520]</ref> |
| − | ISCO can be used to degrade 14D in source areas with a variety of oxidants including permanganate<ref>Waldemer, R.H. and Tratnyek, P.G., 2006. Kinetics of contaminant degradation by permanganate. Environmental Science & Technology, 40(3), pp.1055-1061. [https://doi.org/10.1021/es051330s doi: https://doi.org/10.1021/es051330s]</ref>, persulfate<ref>Félix-Navarro, R.M., Lin-Ho, S.W., Barrera-Díaz, N. and Pérez-Sicairos, S., 2007. Kinetics of the degradation of 1, 4-dioxane using persulfate. Journal of the Mexican Chemical Society, 51(2), pp.67-71. ISSN 1870-249X</ref>, Fenton’s reagent<ref>Kiker, J.H., Connolly, J.B., Murray, W.A., Pearson, S.C.P., Reed, S.E.R. and Tess, R.J., 2010. Ex-situ wellhead treatment of 1, 4-dioxane using fenton's reagent. In Proceedings of the Annual International Conference on Soils, Sediments, Water and Energy (Vol. 15, No. 1, p. 18).</ref> and ozone<ref name= "Ikehata"/>. However, ISCO treatment of 14D in source areas is not common because much of the mass is in the downgradient plume. Dilute plumes can potentially be treated using cylinders placed in wells that slowly release oxidant over time. In a field demonstration, a system of slow release persulfate cylinders was effective in reducing 14D and chlorinated solvent concentrations by over 99% for 119 days<ref>Evans, P., Hooper, J., Lamar, M., Nguyen, D., Dugan, P., Crimi, M. and Ruiz, N., 2018. Sustained In situ Chemical Oxidation (ISCO) of 1, 4 Dioxane and Chlorinated VOCs Using Slow release Chemical Oxidant Cylinders. ESTCP Cost and Performance Report, ER-201324. [[media:2018-Evans-ER-201324_Cost_%26_Performance_Report.pdf| Report.pdf]]</ref>.
| + | |- |
| | + | | Installation of check valves (backflow preventers) in sewer lateral lines connecting residences to the sanitary sewer line containing elevated petroleum vapor concentrations. </br>Note: for VI mitigation, the check valve must control both liquid and vapor flow (e.g., Checkmate inline check valve). || Jarvela et al., 2003<ref>Jarvela, S., Boyd, K. and Gadinski, R., 2003, April. Tranguch Gasoline Site Case History. In International Oil Spill Conference (Vol. 2003, No. 1, pp. 637-642). American Petroleum Institute. [https://doi.org/10.7901/2169-3358-2003-1-637 doi: 10.7901/2169-3358-2003-1-637]</ref> |
| | + | |- |
| | + | | Sewer line ventilation || Riis et al., 2010<ref>Riis, C.E., Christensen, A.G., Hansen, M.H., Husum, H. and Terkelsen, M., 2010, May. Vapor intrusion through sewer systems: Migration pathways of chlorinated solvents from groundwater to indoor air. In Seventh Battelle International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey. [[media:2010-Riis-Migratioin_pathways_of_Chlorinated_Solvents.pdf| Report.pdf]]</ref></br>Nielsen and Hivdberg, 2017<ref name= "Nielsen2017"/></br>ERM, 2017<ref name= "ERM2017"/></br>Holton and Simms, 2018<ref>Holton, C.; Simms, J., 2018. A Review of Preferential Pathway Case Studies: Lessons Learned for Vapor Intrusion Site Assessment. Midwestern States Environmental Consultants Association Spring Seminar, Indianapolis, Indiana. [[media:2018-Holton-A_Review_of_Pref_Path_Case_Studies.pdf| report.pdf]]</ref> |
| | + | |- |
| | + | | Sewer line repair || Viteri et al., 2018<ref>Viteri, C. R.; Richman, B.; Vitouchkine, A.; Armen, M. A.; Miller, A., 2018. Rapid, Real-time TCE Measurements of Sewer Headspace: Characterizing Spatial and Temporal Variability. AEHS 28th Annual International Conference on Soil, Water, Energy, and Air, San Diego [[media:2018-Viteri_Rapid_Real-time_TCE_Measurements.pdf| Report.pdf]] |
| | | | |
| − | Thermal remediation of source areas is not frequently utilized for 14D remediation because much of the 14D mass is in the dissolved plume. However, when applied to treat a source area, thermal remediation can be effective. Following electrical resistive heating of a chlorinated solvent source area, 14D levels in groundwater were reduced >99%<ref>Oberle, D., Crownover, E. and Kluger, M., 2015. In situ remediation of 1, 4‐dioxane using electrical resistance heating. Remediation Journal, 25(2), pp.35-42. [https://doi.org/10.1002/rem.21422 doi: 10.1002/rem.21422]</ref>. 14D source areas can also be treated using a combination of heated air injection and focused air extraction from areas with elevated 14D concentrations. In a field demonstration, thermally enhanced [https://enviro.wiki/index.php?title=Soil_Vapor_Extraction_(SVE) soil vapor extraction] reduced 14D levels by 94%<ref>Hinchee, R.E., P.C. Johnson, P.R. Dahlen, and D.R. Durris, 2017. 1,4-Dioxane remediation by extreme soil vapor extraction (XSVE). Final Report ESTCP Project 201326 [[media:2017-Hinchee-1.4_Dioxane_remediation_by_extreme_soil_XSVE_ER-201326_Final_Report.pdf| Report.pdf]]</ref><ref>Hinchee, R.E., Dahlen, P.R., Johnson, P.C. and Burris, D.R., 2018. 1, 4‐Dioxane Soil Remediation Using Enhanced Soil Vapor Extraction: I. Field Demonstration. Groundwater Monitoring & Remediation, 38(2), pp.40-48. [https://doi.org/10.1111/gwmr.12264 doi: doi.org/10.1111/gwmr.12264]</ref>. Modeling results indicate that removal increases with increasing air injection temperature and relative humidity and decreases with initial soil moisture content<ref>Burris, D.R., Johnson, P.C., Hinchee, R.E. and Dahlen, P.R., 2018. 1, 4‐Dioxane Soil Remediation Using Enhanced Soil Vapor Extraction (XSVE): II. Modeling. Groundwater Monitoring & Remediation, 38(2), pp.49-56. [https://doi.org/10.1111/gwmr.12277 doi: 10.1111/gwmr.12277]</ref>.
| |
| | | | |
| − | ''In situ'' bioremediation has not been commonly applied to 14D treatment due to the slow growth of bacteria using 14D as a growth substrate (see [Biodegradation – 1,4-Dioxane]). However, there has been some success in stimulating cometabolic biodegradation of 14D using gaseous substrates. In a biosparging pilot test, air amended with propane was injected into a single well that was bioaugmented with the propanotroph ''Rhodococcus ruber'' ENV425. 14D levels decreased by >99% in the sparge well and two nearby monitor wells<ref name= "Lippincott2015"/>. A propane-stimulated groundwater recirculation system has also recently been reported to effectively reduce concentrations of 14D as well as several chlorinated aliphatics such as TCE and 1,2-dichloroethane<ref name= "Chu2018"/>.
| |
| | | | |
| − | Phytoremediation has been reported as a viable treatment alternative for 14D<ref>Aitchison, E.W., Kelley, S.L., Alvarez, P.J. and Schnoor, J.L., 2000. Phytoremediation of 1, 4-dioxane by hybrid poplar trees. Water Environment Research, 72(3), pp.313-321. [https://doi.org/10.2175/106143000X137536 doi: 10.2175/106143000X137536]</ref>. The primary removal process is via phytovolatilization, which involves transpiration of chemicals from the leaf surfaces to the atmosphere<ref>Ouyang, Y., 2002. Phytoremediation: modeling plant uptake and contaminant transport in the soil–plant–atmosphere continuum. Journal of Hydrology, 266(1-2), pp.66-82. [https://doi.org/10.2175/106143000X137536 doi: 10.2175/106143000X137536]</ref>. Addition of 14D degrading bacteria (e.g. CB1190) and appropriate co-substrates can further enhance the removal of 14D in soil planted with poplar trees<ref>Kelley, S.L., Aitchison, E.W., Deshpande, M., Schnoor, J.L. and Alvarez, P.J., 2001. Biodegradation of 1, 4-dioxane in planted and unplanted soil: effect of bioaugmentation with Amycolata sp. CB1190. Water Research, 35(16), pp.3791-3800. [https://doi.org/10.1016/S0043-1354(01)00129-4 doi: 10.1016/S0043-1354(01)00129-4]</ref>.
| + | ==Summary== |
| | + | Storm and sanitary sewers, electrical and communications conduits, and other tunnels can serve as preferential pathways, in some cases allowing vapor to enter buildings. Sites at higher risk for VI from preferential pathways include sites where contaminated groundwater enters the sewer or where the conduit passes through a concentrated VOC source area. Monitoring results indicate that sewer to indoor air attenuation is similar in magnitude to sub-slab to indoor air attenuation, suggesting that sub-slab screening values can also be used to evaluate sewer vapor results. Mitigation approaches from sewers and tunnels include preventing contaminants from entering the conduit, ventilating the line, or correcting plumbing defects. |
| | | | |
| − | Early microcosm studies indicated that 14D did not degrade in the presence of impacted aquifer material under ''in situ'' conditions<ref name= "Zenker 2000"/>. However, a statistical analysis of multiple Air Force and California sites found evidence of 14D removal in 19% of the sites<ref name= "Adamson2015">Adamson, D.T., Anderson, R.H., Mahendra, S. and Newell, C.J., 2015. Evidence of 1, 4-dioxane attenuation at groundwater sites contaminated with chlorinated solvents and 1, 4-dioxane. Environmental Science & Sechnology, 49(11), pp.6510-6518.[ https://doi.org/10.1021/acs.est.5b00964 doi:10.1021/acs.est.5b00964]</ref>, similar to other chlorinated VOCs (TCE and 1,1-DCE). The decay rates were positively correlated to dissolved oxygen, and negatively correlated to high metals and chlorinated VOC concentrations<ref name= "Adamson2015"/>. 14D decay has also been positively correlated to the presence of oxidizing enzymes<ref name= "Li2013">Li, M., Mathieu, J., Yang, Y., Fiorenza, S., Deng, Y., He, Z., Zhou, J. and Alvarez, P.J., 2013. Widespread distribution of soluble di-iron monooxygenase (SDIMO) genes in arctic groundwater impacted by 1, 4-dioxane. Environmental science & technology, 47(17), pp.9950-9958. [https://doi.org/10.1021/es402228x doi: 10.1021/es402228x]</ref><ref>Gedalanga, P.B., Pornwongthong, P., Mora, R., Chiang, S.Y.D., Baldwin, B., Ogles, D. and Mahendra, S., 2014. Identification of biomarker genes to predict biodegradation of 1, 4-dioxane. Appl. Environ. Microbiol., 80(10), pp.3209-3218. [https://doi.org/10.1128/aem.04162-13 doi: 10.1128/AEM.04162-13]</ref>. Native 14D degrading microorganisms have also been detected in field studies using a number of environmental diagnostic assays, such as microarray<ref name= "Li2013"/> and stable isotope probing<ref>Chiang, S.Y.D., Mora, R., Diguiseppi, W.H., Davis, G., Sublette, K., Gedalanga, P. and Mahendra, S., 2012. Characterizing the intrinsic bioremediation potential of 1, 4-dioxane and trichloroethene using innovative environmental diagnostic tools. Journal of Environmental Monitoring, 14(9), pp.2317-2326. [https://doi.org/10.1039/c2em30358b doi: 10.1039/C2EM30358B]</ref><ref>Li, M., Liu, Y., He, Y., Mathieu, J., Hatton, J., DiGuiseppi, W. and Alvarez, P.J., 2017. Hindrance of 1, 4-dioxane biodegradation in microcosms biostimulated with inducing or non-inducing auxiliary substrates. Water Research, 112, pp.217-225. [http://dx.doi.org/10.1016/j.watres.2017.01.047 doi: 10.1016/j.watres.2017.01.047]</ref>, suggesting that monitored natural attenuation (MNA) may be a feasible and cost-efficient alternative to mitigate 14D at some sites.
| |
| | | | |
| | ==References== | | ==References== |