Difference between revisions of "User:Jhurley/sandbox"
(→Regulatory Drivers for Transition to PFAS Free Firefighting Formulations) |
|||
| Line 49: | Line 49: | ||
==Regulatory Drivers for Transition to PFAS Free Firefighting Formulations== | ==Regulatory Drivers for Transition to PFAS Free Firefighting Formulations== | ||
| − | Regulations restricting the use and release of PFAS are being proposed and promulgated worldwide, with several enacted regulations addressing the use of aqueous film forming foams (AFFF) containing PFAS<ref name="Queensland2016">Queensland (Australia) Department of Environment and Heritage Protection, 2016. Operational Policy - Environmental Management of Firefighting Foam. 16 pages. [https://environment.des.qld.gov.au/assets/documents/regulation/firefighting-foam-policy.pdf Free Download]</ref><ref>U.S. Congress, 2019. S.1790 - National Defense Authorization Act for Fiscal Year 2020. United States Library of Congress. [https://www.congress.gov/bill/116th-congress/senate-bill/1790 Text and History of Law].</ref><ref>Arizona State Legislature, 2019. Title 36, Section 1696. Firefighting foam; prohibited uses; exception; definitions. [https://www.azleg.gov/viewdocument/?docName=https://www.azleg.gov/ars/36/01696.htm Text of Law]</ref><ref>California Legislature, 2020. Senate Bill No. 1044, Chapter 308, Firefighting equipment and foam: PFAS chemicals. [https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201920200SB1044 Text and History of Law]</ref>ref>Arkansas General Assembly, 2021. An Act Concerning the Use of Certain Chemicals in Firefighting Foam; and for Other Purposes. Act 315, State of Arkansas. [https://trackbill.com/bill/arkansas-house-bill-1351-concerning-the-use-of-certain-chemicals-in-firefighting-foam/2008913/ Text and History of Law].</ref><ref>Espinosa, Summers, Kelly, J., Statler, Hansen, Young, 2021. Amendment to Fire Prevention and Control Act. House Bill 2722. West Virginia Legislature. [https://trackbill.com/bill/west-virginia-house-bill-2722-prohibiting-the-use-of-class-b-fire-fighting-foam-for-testing-purposes-if-the-foam-contains-a-certain-class-of-fluorinated-organic-chemicals/2047674/ Text and History of Law]</ref><ref>Louisiana Legislature, 2021. Act No. 232. [https://trackbill.com/bill/louisiana-house-bill-389-fire-protect-fire-marshal-provides-relative-to-the-discharge-or-use-of-class-b-fire-fighting-foam-containing-fluorinated-organic-chemicals/2092535/ Text and History of Law]</ref><ref>Vermont Legislature, 2021b. Act No. 36, PFAS in Class B Firefighting Foam. [https://trackbill.com/bill/vermont-senate-bill-20-an-act-relating-to-restrictions-on-perfluoroalkyl-and-polyfluoroalkyl-substances-and-other-chemicals-of-concern-in-consumer-products/1978963/ History and Text of Law]</ref>. In addition to regulated usage, firefighting formulation users are transitioning to PFAS-free firefighting formulations to reduce environmental liability in the event of a release, to reduce the cost of expensive containment systems and management of generated waste streams, and to avoid reputational damage. In 2016, Queensland, Australia was one of the first governments to ban PFAS use in firefighting foam<ref name="Queensland2016"/>. The US 2020 National Defense Authorization Act specifies immediate prohibition of controlled releases of AFFF containing PFAS and requires the Secretary of the Navy to publish a specification for PFAS-free firefighting formulation use and ensure it is available for use by the Department of Defense (DoD) by October 1, 2023<ref>U.S. Congress, 2021. S.2792 - National Defense Authorization Act for Fiscal Year 2021. United States Library of Congress. [https://www.congress.gov/bill/117th-congress/senate-bill/2792/ Text and History of Law].</ref>. The National Fire Protection Association (NFPA) recently removed the requirement for AFFF containing PFAS from their Standard on Aircraft Hangars and added two new chapters to allow users to determine if AFFF containing PFAS is needed at their facility<ref name="NFPA2022">National Fire Protection Association (NFPA), 2022. Codes and Standards, 409: Standard on Aircraft Hangars. [https://www.nfpa.org/codes-and-standards/4/0/9/409?l=42 NFPA Website]</ref>. | + | Regulations restricting the use and release of PFAS are being proposed and promulgated worldwide, with several enacted regulations addressing the use of aqueous film forming foams (AFFF) containing PFAS<ref name="Queensland2016">Queensland (Australia) Department of Environment and Heritage Protection, 2016. Operational Policy - Environmental Management of Firefighting Foam. 16 pages. [https://environment.des.qld.gov.au/assets/documents/regulation/firefighting-foam-policy.pdf Free Download]</ref><ref>U.S. Congress, 2019. S.1790 - National Defense Authorization Act for Fiscal Year 2020. United States Library of Congress. [https://www.congress.gov/bill/116th-congress/senate-bill/1790 Text and History of Law].</ref><ref>Arizona State Legislature, 2019. Title 36, Section 1696. Firefighting foam; prohibited uses; exception; definitions. [https://www.azleg.gov/viewdocument/?docName=https://www.azleg.gov/ars/36/01696.htm Text of Law]</ref><ref>California Legislature, 2020. Senate Bill No. 1044, Chapter 308, Firefighting equipment and foam: PFAS chemicals. [https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201920200SB1044 Text and History of Law]</ref><ref>Arkansas General Assembly, 2021. An Act Concerning the Use of Certain Chemicals in Firefighting Foam; and for Other Purposes. Act 315, State of Arkansas. [https://trackbill.com/bill/arkansas-house-bill-1351-concerning-the-use-of-certain-chemicals-in-firefighting-foam/2008913/ Text and History of Law].</ref><ref>Espinosa, Summers, Kelly, J., Statler, Hansen, Young, 2021. Amendment to Fire Prevention and Control Act. House Bill 2722. West Virginia Legislature. [https://trackbill.com/bill/west-virginia-house-bill-2722-prohibiting-the-use-of-class-b-fire-fighting-foam-for-testing-purposes-if-the-foam-contains-a-certain-class-of-fluorinated-organic-chemicals/2047674/ Text and History of Law]</ref><ref>Louisiana Legislature, 2021. Act No. 232. [https://trackbill.com/bill/louisiana-house-bill-389-fire-protect-fire-marshal-provides-relative-to-the-discharge-or-use-of-class-b-fire-fighting-foam-containing-fluorinated-organic-chemicals/2092535/ Text and History of Law]</ref><ref>Vermont Legislature, 2021b. Act No. 36, PFAS in Class B Firefighting Foam. [https://trackbill.com/bill/vermont-senate-bill-20-an-act-relating-to-restrictions-on-perfluoroalkyl-and-polyfluoroalkyl-substances-and-other-chemicals-of-concern-in-consumer-products/1978963/ History and Text of Law]</ref>. In addition to regulated usage, firefighting formulation users are transitioning to PFAS-free firefighting formulations to reduce environmental liability in the event of a release, to reduce the cost of expensive containment systems and management of generated waste streams, and to avoid reputational damage. In 2016, Queensland, Australia was one of the first governments to ban PFAS use in firefighting foam<ref name="Queensland2016"/>. The US 2020 National Defense Authorization Act specifies immediate prohibition of controlled releases of AFFF containing PFAS and requires the Secretary of the Navy to publish a specification for PFAS-free firefighting formulation use and ensure it is available for use by the Department of Defense (DoD) by October 1, 2023<ref>U.S. Congress, 2021. S.2792 - National Defense Authorization Act for Fiscal Year 2021. United States Library of Congress. [https://www.congress.gov/bill/117th-congress/senate-bill/2792/ Text and History of Law].</ref>. The National Fire Protection Association (NFPA) recently removed the requirement for AFFF containing PFAS from their Standard on Aircraft Hangars and added two new chapters to allow users to determine if AFFF containing PFAS is needed at their facility<ref name="NFPA2022">National Fire Protection Association (NFPA), 2022. Codes and Standards, 409: Standard on Aircraft Hangars. [https://www.nfpa.org/codes-and-standards/4/0/9/409?l=42 NFPA Website]</ref>. |
==Selection of Replacement PFAS Free Firefighting Formulations== | ==Selection of Replacement PFAS Free Firefighting Formulations== | ||
Revision as of 20:35, 27 March 2024
Transition of Aqueous Film Forming Foam (AFFF) Fire Suppression Infrastructure Impacted by Per and Polyfluoroalkyl Substances (PFAS)
Per and polyfluoroalkyl substances (PFAS) contained in Class B aqueous film-forming foams (AFFFs) are known to accumulate on wetted surfaces of many fire suppression systems after decades of exposure[1]. When replacement PFAS-free firefighting formulations are added to existing infrastructure, PFAS can rebound from the wetted surfaces into the new formulations at high concentrations[2][3]. Effective methods are needed to properly transition to PFAS-free firefighting formulations in existing fire suppression infrastructure. Considerations in the transition process may include but are not limited to locating, identifying, and evaluating existing systems and AFFF, fire engineering evaluations, system prioritization, cost/downtime analyses, sampling and analysis, evaluation of risks and hazards to human health and the environment, transportation, and disposal.
Contents
- 1 Transition of Aqueous Film Forming Foam (AFFF) Fire Suppression Infrastructure Impacted by Per and Polyfluoroalkyl Substances (PFAS)
- 2 Introduction
- 3 PFAS Assembly on Solid Surfaces
- 4 Thermodynamics of PFAS Accumulations on Solid Surfaces
- 5 Regulatory Drivers for Transition to PFAS Free Firefighting Formulations
- 6 Selection of Replacement PFAS Free Firefighting Formulations
- 7 Hydrogeophysical Technologies for Understanding Groundwater-Surface Water Interactions
- 8 Guidelines for Implementing Hydrogeophysical Methods into Groundwater/Surface Water Interaction Studies
- 9 Summary
- 10 References
- 11 See Also
Related Article(s):
- Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS)
- PFAS Sources
- PFAS Ex Situ Water Treatment
- Supercritical Water Oxidation (SCWO)
- PFAS Treatment by Electrical Discharge Plasma
Contributor(s):
- Dr. Johnsie Ray Lang
- Dr. Jonathan Miles
- John Anderson
- Dr. Theresa Guillette
- Dr. Craig Divine
- Dr. Stephen Richardson
Key Resource(s):
- Department of Defense (DoD) performance standard for PFAS-free firefighting formulation: Military Specification MIL-PRF-32725[4]
- Characterization of per- and polyfluoroalkyl substances on fire suppression system piping and optimization of removal methods[1]
Introduction
PFAS are a class of synthetic fluorinated compounds which are highly mobile and persistent within the environment[5]. Due to the surfactant properties of PFAS, these compounds self-assemble at any solid-liquid interface forming resilient bilayers during prolonged exposure[6]. Solid phase accumulation of PFAS has been proposed to be influenced by both hydrophobic and electrostatic interactions with fluorinated carbon chain length as the dominant feature influencing sorption[7]. While the majority of previous research into solid phase sorption typically focused on water treatment applications or subsurface porous media[8], recently PFAS accumulations have been demonstrated on the wetted surfaces of fire suppression infrastructure exposed to aqueous film forming foam (AFFF)[1] (see Figure 1).
Fire suppression systems with potential PFAS impacts include fire fighting vehicles that carried AFFF and fixed suppression systems in buildings containing large amounts of flammable materials such as aircraft hangars (Figure 2). PFAS residue on the wetted surfaces of existing infrastructure can rebound into replacement PFAS-free firefighting formulations if not removed during the transition process[2]. Simple surface rinsing with water and low-pressure washing has been proven to be inefficient for removal of surface bound PFAS from piping and tanks that contained fluorinated AFFF[2]
In addition to proper methods for system cleaning to remove residual PFAS, transition to PFAS-free foam may also include consideration of compliance with state and federal regulations, selection of the replacement PFAS free firefighting formulation, a cost benefit analysis for replacement of the system components versus cleaning, and PFAS verification testing. Foam transition should be completed in a manner which minimizes the volume of waste generated as well as preventing any PFAS release into the environment.
PFAS Assembly on Solid Surfaces
The self-assembly of amphiphilic molecules into supramolecular bilayers is a result of their structure and how it interacts with the bulk water of a solution. Single chain hydrocarbon based amphiphiles can form micelles under relatively dilute aqueous concentrations, however for hydrocarbon based surfactants the formation of more complex organized system such as vesicles is rarely seen, requiring double chain amphiphiles such as phospholipids. Associations of single chain cationic and anionic hydrocarbon based amphiphiles into stable supramolecular structures such as vesicles has however been demonstrated[9], with the ion pairing of the polar head groups mimicking the a double tail situation. The behavior of single chain fluorosurfactant amphiphiles has been demonstrated to be significantly different from similar hydrocarbon based analogues. Not only are critical micelle concentrations (CMC) of fluorosurfactants typically two orders of magnitude lower than corresponding hydrocarbon surfactants but self-assembly can occur even when fluorosurfactants are dispersed at low concentrations significantly below the CMC in water and other solvents[10]. The assembly of fluorinated amphiphiles structurally similar to those found in AFFF have been shown to readily form stable, complex structures including vesicles, fibers, and globules at concentrations as low as 0.5% w/v in contrast to their hydrocarbon analogues which remained fluid at 30% w/v[11][12].
Krafft found that fluorinated amphiphiles formed bilayer membranes with phospholipids, and that the resulting vesicles were more stable than those made of phospholipids alone[13]. The similarities in amphiphilic properties between phospholipids and the hydrocarbon-based surfactants in AFFF suggests that bilayer vesicles may form between these and the fluorosurfactants also present in the concentrate. Krafft demonstrated that both the permeability of resulting mixed vesicles and their propensity to fuse with each other at increasing ionic strength was reduced as a result of the creation of an inert hydrophobic and lipophobic film within the membrane, and also suggested that the fluorinated amphiphiles increased van der Waals interactions in the hydrocarbon region[13]. Thus this low permeability may allow vesicles formed by the surfactants present in AFFF to act as long term repositories of PFAS not only as part of the bilayer itself but also solvated within the vesicle. This prediction is supported by the observation that supramolecular structures formed from single chain fluorinated amphiphiles have been demonstrated to be stable at elevated temperature (15 min at 121°C) and have been shown to be stable over periods of months, even increasing in size over time when stored at environmentally relevant temperatures[12].
Formation of complex structures at relatively low solute concentrations requires the monomer molecules to be well ordered to maintain tight packing in the supramolecular structure[14]. This order results from electrostatic forces, hydrogen bonding, and in the case of fluorinated amphiphiles, hydrophobic interactions. The geometry of the amphiphile also potentially contributes to the type of supramolecular aggregation[15]. Surfactants which adopt a conical shape (such as a typical hydrocarbon based surfactant with a large polar head group and a single alkyl chain as a tail) tend to form micelles more easily. Increasing the bulk of the tail makes the surfactant more cylindrically shaped which makes assembly into bilayers more likely.
Perfluoroalkyl chains are significantly more bulky than their hydrocarbon based analogues both in cross sectional area (28-30 Å2 versus 20 Å2, respectively) and mean volume (CF2 and CF3 estimated as 38 Å3 and 92 Å3 compared to 27 Å3 and 54 Å3 for CH2 and CH3)[13][10]. Structural studies on linear PFOS have shown that the molecule adopts an unusual helical structure[16][17] in aqueous and solvent phases to alleviate steric hindrance. This arrangement results from the carbon chain starting in the planar all anti conformation and then successively twisting all the CC-CC dihedrals by 15°-20° in the same direction[18]. The conformation also minimizes the electrostatic repulsion between fluorine atoms bonded to the same side of the carbon backbone by maximizing the interatomic distances between them[17].
A consequence of the helical structure is that there is limited carbon-carbon bond rotation within the perfluoroalkyl chain giving them increased rigidity compared to alkyl chains[19]. The bulkiness of the perfluoroalkyl chain confers a cylindrical shape on the fluorosurfactant amphiphile and therefore favors the formation of bilayers and vesicles the aggregation of which is further assisted by the rigidity of the molecules which allow close packing in the supramolecular structure. Fluorosurfactants therefore cannot be regarded as more hydrophobic analogues of hydrogenated surfactants. Their self-assembly behavior is characterized by a strong tendency to form vesicles and lamellar phases rather than micelles, due to the bulkiness and rigidity of the perfluoroalkyl chain that tends to decrease the curvature of the aggregates they form in solution[20]. The larger tail cross section of fluorinated compared to hydrogenated amphiphiles tends to favor the formation of aggregates with lesser surface curvature, therefore rather than micelles they form bilayer membranes, vesicles, tubules and fibers[21][22][23][24]. Rojas et al. (2002) demonstrated that perfluorooctyl sulphonamide formed a contiguous bilayer at 50 mg/L with self-assembled aggregates present at concentrations as low as 10 mg/L[25].
Thermodynamics of PFAS Accumulations on Solid Surfaces
The thermodynamics of formation of amphiphiles into supramolecular species requires consideration of both hydrophobic and hydrophilic interactions resulting from the amphoteric nature of the molecule. The hydrophilic portions of the molecule are driven to maximize their solvation interaction with as many water molecules as possible, whereas the hydrophobic portions of the molecule are driven to aggregate together thus minimizing interaction with the bulk water. Both of these processes change the enthalpy and entropy of the system.
In aqueous solution, the hydrophilic portions of an amphiphile form hydrogen bonds (4 - 120 kJ/mol) and van der Waals interactions (<5 kJ/mol) with water molecules and surfaces, and electrostatic interactions (5 – 300 kJ/mol) can also occur where the amphiphile is ionic[26]. These interactions, although weak compared to intramolecular covalent bonds within a molecule are energetically favorable and increase the enthalpy of the combined solute-solvent system. Thus, the hydrophilic portion of an amphiphile will look to maximize enthalpic gain through hydrogen bond interactions with the bulk water.
The hydrophobic portion of an amphiphile cannot form hydrogen bonds with the bulk solution, and its presence disrupts the hydrogen bond interactions between individual water molecules within the bulk water matrix. This disruption lowers the entropy of the system by reducing the degrees of translational rotational freedom available to the bulk water. The second law of thermodynamics dictates that a system will arrange itself to maximize its entropy. With hydrophobic species this can be achieved by their spontaneous aggregation, as the reduction in solution entropy of the aggregated system is less than that which would occur if the component parts were solvated individually. These hydrophobic and hydrophilic interactions are weak, and the individual entropy gain per amphiphile upon aggregation is very small. However, taken together the overall effect on the entropy of the aggregate is sufficient to maintain it in solution, and moreover these interactions make the aggregates resistant to minor perturbations while retaining the reversibility of the self-assembled structure[26].
Regulatory Drivers for Transition to PFAS Free Firefighting Formulations
Regulations restricting the use and release of PFAS are being proposed and promulgated worldwide, with several enacted regulations addressing the use of aqueous film forming foams (AFFF) containing PFAS[27][28][29][30][31][32][33][34]. In addition to regulated usage, firefighting formulation users are transitioning to PFAS-free firefighting formulations to reduce environmental liability in the event of a release, to reduce the cost of expensive containment systems and management of generated waste streams, and to avoid reputational damage. In 2016, Queensland, Australia was one of the first governments to ban PFAS use in firefighting foam[27]. The US 2020 National Defense Authorization Act specifies immediate prohibition of controlled releases of AFFF containing PFAS and requires the Secretary of the Navy to publish a specification for PFAS-free firefighting formulation use and ensure it is available for use by the Department of Defense (DoD) by October 1, 2023[35]. The National Fire Protection Association (NFPA) recently removed the requirement for AFFF containing PFAS from their Standard on Aircraft Hangars and added two new chapters to allow users to determine if AFFF containing PFAS is needed at their facility[36].
Selection of Replacement PFAS Free Firefighting Formulations
Since they first entered the market in the 2000s, the operational capabilities of PFAS free firefighting formulations have grown
Hydrogeophysical Technologies for Understanding Groundwater-Surface Water Interactions
Hydrogeophysical technologies exploit contrasts in the physical properties between groundwater and surface water to detect and monitor zones of pronounced GWSWE. The two most valuable properties to measure are temperature and electrical conductivity. Temperature has been used for decades as an indicator of groundwater-surface water exchange[37] with early uses including pushing a thermistor into the bed of a surface water body to assess zones of surface water downwelling and groundwater upwelling. Today, a variety of novel technologies that measure temperature over a wide range of spatial and temporal scales are being used to investigate GWSWE. The evaluation of electrical conductivity measurements using point probes and geophysical imaging is also well-established. However, new technologies are now available to exploit electrical conductivity contrasts from GWSWE occurring over a range of spatial and temporal scales.
Temperature-Based Technologies
Several temperature-based GWSWE methodologies exploit the gradient in temperature between surface water and groundwater that exist during certain times of day or seasons of the year. The thermal insulation provided by the Earth’s land surface means that groundwater is warmer than surface water in winter months, but colder than surface water in summer months away from the equator. Therefore, in temperate climates, localized (or ‘preferential’) groundwater discharge into surface water bodies is often observed as cold temperature anomalies in the summer and warm temperature anomalies in the winter. However, there are times of the year such as fall and spring when contrasts in the temperature between groundwater and surface water will be minimal, or even undetectable. These seasonal-driven points in time correspond to the switch in the polarity of the temperature contrast between groundwater and surface water. Consequently, SW to GW gradient temperature-based methods are most effective when deployed at times of the year when the temperature contrasts between groundwater and surface water are greatest. Other time-series temperature monitoring methods depend more on natural daily signals measured at the bed interface and in bed sediments, and those signals may exist year round except where strongly muted by ice cover or surface water stratification. A variety of sensing technologies now exist within the GWSWE toolbox, from techniques that rapidly characterize temperature contrasts over large areas, down to powerful monitoring methods that can continuously quantify GWSWE fluxes at discrete locations identified as hotspots.
Characterization Methods
The primary use of the characterization methods is to rapidly determine precise locations of groundwater upwelling over large areas in order to pinpoint locations for subsequent ground-based observations. A common limitation of these methods is that they can only sense groundwater fluxes into surface water. Methods applied at the water surface and in the surface water column generally cannot detect localized regions of surface water transfer to groundwater, for which temperature measurements collected within the bed sediments are needed. This is a more challenging characterization task that may, in the right conditions, be addressed using electrical conductivity-based methods described later in this article.
Unmanned Aerial Vehicle Infrared (UAV-IR)
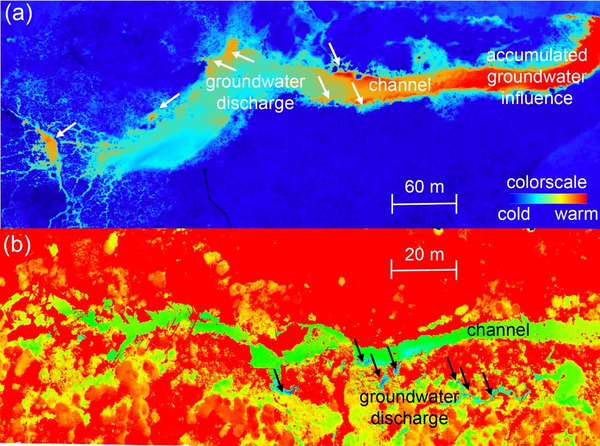
Unmanned aerial vehicles (UAVs) equipped with thermal infrared (IR) cameras can provide a very powerful tool for rapidly determining zones of pronounced upwelling of groundwater to surface water. Large areas of can be covered with high spatial resolution. The information obtained can be used to rapidly define locations of focused groundwater upwelling and prioritize these for more intensive surface-based observations (Figure 1). As with all thermal methods, flights must be performed when adequate contrasts in temperature between surface water and groundwater are expected to exist. Not just time of year but, because of the effect of the diurnal temperature signal on surface water bodies, time of day might need to be considered in order to maximize the chance of success. Calibration of UAV-IR camera measurements against simultaneously acquired direct measurements of temperature is recommended to optimize the value of these datasets. UAV-IR methods will not work in all situations. One major limitation of the technology is that the temperature expression of groundwater upwelling must be manifested at the surface of the surface water body. Consequently, the technology will not detect relatively small discharges occurring beneath a relatively deep surface water layer, and thermal imaging over the water surface can be complicated by thermal IR reflection. The chances of success with UAV-IR will be strongest in regions of exposed banks or shallow water where there are no strong currents causing mixing (and thus dilution) of the upwelling groundwater temperature signals. UAV-IR methods will therefore likely be most successful close to shorelines of lakes/ponds, over shallow, low flow streams and rivers and in wetland environments. UAV-IR methods require a licensed pilot, and restrictions on the use of airspace may limit the application of this technology.
Handheld Thermal Infrared (TIR) Cameras
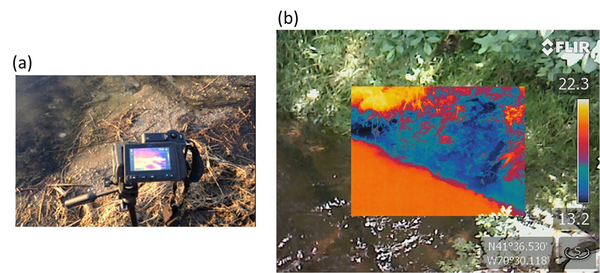
Hand-held thermal infrared (TIR) cameras are powerful tools for visual identification of localized seeps of upwelling groundwater. TIR cameras may be used to follow up on UAV-IR surveys to better characterize local seeps identified from the air using UAV-IR. Alternatively, a TIR camera is a valuable tool when performing initial walks of prospective study sites as they may quickly confirm the presence of suspected seeps. TIR cameras provide high resolution images that can define the structure of localized seeps and may provide valuable insights into the role of discrete features (e.g., fractures in rocks or pipes in soil) in determining seep morphology (Figure 2). Like UAV-IR, TIR provides primarily qualitative information (location, extent) of seeps and it only succeeds when there are adequate contrasts between groundwater and surface water that are expressed at the surface of the investigated water body or along bank sediments. The United States Geological Survey (USGS) has made extensive use of TIR cameras for studying groundwater/surface-water exchange.
Continuous Near-bed Temperature Sensing
When performing surveys from a boat a simple yet often powerful technology is continuous near-bed temperature sensing, whereby a temperature probe is strategically suspended to float in the water column just above the bed or dragged along it. Compared to UAV-IR, this approach does not rely on upwelling groundwater being expressed as a temperature anomaly at the surface. The utility of the method can be enhanced when a specific conductance probe is co- located with the temperature probe so that anomalies in both temperature and specific conductance can be investigated.
Monitoring Methods
Monitoring methods allow temperature signals to be recorded with high temporal resolution along the bed interface or within bank or bed sediments. These methods can capture temporal trends in GWSWE driven by variations in the hydraulic gradients around surface water bodies, as well as changes in hydraulic conductivity due to sedimentation, clogging, scour or microbial mass. If vertical profiles of bed temperature are collected, a range of analytical and numerical models can be applied to infer the vertical water flux rate and direction, similar to a seepage meter. These fluxes may vary as a function of season, rainfall events (enhanced during storm activity), tidal variability in coastal settings and due to engineered controls such as dam discharges. The methods can capture evidence of GWSWE that may not be detected during a single ‘characterization’ survey if the local hydraulic conditions at that point in time result in relatively weak hydraulic gradients.
Fiber-optic Distributed Temperature Sensing (FO-DTS)
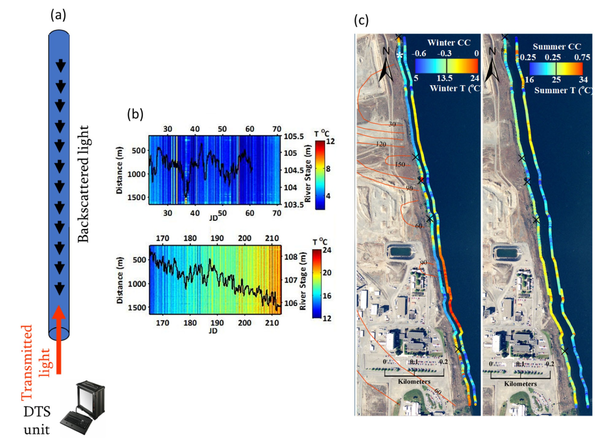
Fiber-optic distributed temperature sensing (FO-DTS) is a powerful monitoring technology used in fire detection, industrial process monitoring, and petroleum reservoir monitoring. The method is also used to obtain spatially rich datasets for monitoring GWSWE[43][44]. The sensor consists of standard telecommunications fiber-optic fiber typically housed in armored cable and the physics is based on temperature-dependent backscatter mechanisms including Brillouin and Raman backscatter[43]. Most commercially available systems are based on analysis of Raman scatter. As laser light is transmitted down the fiber-optic cable, light scatters continuously back toward the instrument from all along the fiber, with some of the scattered light at frequencies above and below the frequency of incident light, i.e., anti-Stokes and Stokes-Raman backscatter, respectively. The ratio of anti-Stokes to Stokes energy provides the basis for FO-DTS measurements. Measurements are localized to a section of cable according to a time-of-flight calculation (i.e., optical time-domain reflectometry). Assuming the speed of light within the fiber is constant, scatter collected over a specific time window corresponds to a specific spatial interval of the fiber. Although there are tradeoffs between spatial resolution, thermal precision, and sampling time, in practice it is possible to achieve sub meter-scale spatial and approximate 0.1°C thermal precision for measurement cycle times on the order of minutes and cables extending several kilometers[44]; thus, thousands of temperature measurements can be made simultaneously along a single cable. The method allows the visualization of a large amount of temperature data and rapid identification of major trends in GWSWE. Figure 3 illustrates the use of FO-DTS to detect and monitor zones of focused groundwater discharge along a 1.5 km reach of the Columbia River that is threatened by contaminated groundwater[42]. As temperature is only sensed at the cable on the bed, FO-DTS can only detect groundwater inputs to surface water. It cannot detect losses of surface water to groundwater. The USGS public domain software tool DTSGUI allows a user to import, manage, visualize and analyze FO-DTS datasets.
Vertical Temperature Profilers (VTPs)
Analysis methods now allow for the accurate quantification of groundwater fluxes over time. Vertical temperature profilers (VTPs) are sensors applied for diurnal temperature data collection within saturated geologic matrices (Figure 4). Extensive experience with VTPs indicates that the methodology is equal to traditional seepage meters in terms of flux accuracy[45]. However, VTPs have the advantage of measuring continuous temporal variations in flux rates while such information is impractical to obtain with traditional seepage meters.
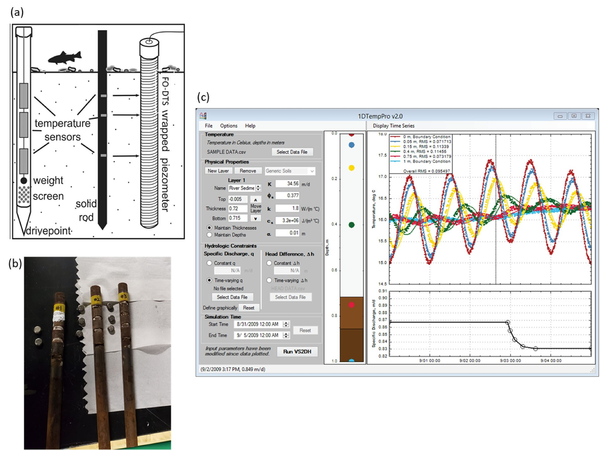
The low-cost design, ease of data collection, and straightforward interpretation of the data using open-source software make VTP sensors increasingly attractive for quantifying flux rates. These sensors typically consist of at least two temperature loggers installed within a steel or plastic pipe filled with foam insulation[46] although the use of loggers installed in well screens or FO-DTS cable wrapped around a piezometer casing (for high vertical resolution data) are also possible (Figure 4a). Loggers are inserted into the insulated housing at different depths, typically starting from one centimeter within the geologic matrix of interest[48]. Temperature loggers usually remain within the first 0.2-meters of the geologic matrix based on the observed limits of diurnal signal influence[49], though zones of strong surface water downwelling may necessitate deeper temperature data collection. Reliability of flux values generated from the temperature signal analysis is dependent in part on the temperature logger precision, VTP placement, sediment heterogeneity, flow direction, flow magnitude[48], and absence of macropore flow. Application of single dimension temperature-based fluid flux models assumes that all flow is vertical and therefore lateral flow within upwelling systems cannot be quantified using VTPs, emphasizing the importance of the VTP installation location over the active area of exchange[48] at shallow depths. Thermal parameters of the geologic matrix where the VTP is installed can be measured using a thermal properties analyzer to record heat capacity and thermal conductivity for later analytical and numerical modeling.
Analytical and numerical solutions, used to solve or estimate the advection-conduction equation within the geologic matrix (bed sediments), continue to evolve to better quantify flux values over time. Analytical solutions to the heat transport equation are used to solve for flux values between sensor pairs from VTP datasets[50][51]. VFLUX is an open-source MATLAB package that allows the user to solve for flux values from a VTP dataset using a variety of analytical solutions[50][51] based on the vertical propagation of diurnal temperature signals. Other emerging ‘spectral’ methods make use of a wide range of natural temperature signals to estimate vertical flux and bed sediment thermal diffusivity[52]. VFLUX analytical solutions are limited by subsurface heterogeneity and diurnal temperature signal strength[48]. 1DTempPro (Figure 4c) provides a graphical user interface (GUI) for numerical solutions to heat transport[53] and does not depend on diurnal signals. Numerical models can produce more accurate flux estimates in the case of complex boundary conditions and abrupt changes in flux rates, but require significant user calibration efforts for longer time series[54]. A hybrid approach between the analytical and numerical solutions, known as tempest1d[54] improves flux modeling with enhanced computational efficiency, resolution of abrupt changes, evaluation of complex boundary conditions, and uncertainty estimations with each step. This new state-space modeling approach uses recursive estimation techniques to automatically estimate highly dynamic vertical flux patterns ranging from sub-daily to seasonal time scales[54].
Electrical Conductivity (EC) Based Technologies
The electrical conductivity (EC)-based technologies exploit contrasts in EC between surface water and groundwater[55]. EC-based technologies are mostly applied as characterization tools, although the opportunity to monitor GWSWE dynamics with one of these technologies does exist. With the exception of specific conductance probes, the technologies measure the bulk EC of sediments, which will often (but not always) reveal evidence of GWSWE.
Electrical conduction (i.e., the transport of charges) in the Earth occurs via the ions dissolved in groundwater, with an additional contribution from ions in the electrical double layer (known as surface conduction)[56]. In relatively fresh surface water environments, groundwater is typically more electrically conductive than surface water due to the higher ion concentrations in groundwater. In these settings, groundwater inputs may be identified as zones of higher bulk EC beneath the bed. In coastal settings where surface water is saline, inputs of relatively fresh groundwater will give rise to zones of lower conductivity. Whereas the temperature-based methods rely on point measurements at the location of the sensor, the EC-based technologies (with the exception of point specific conductance measurements) incorporate inverse modeling to estimate distributions of EC away from the sensors and beneath the bed. Consequently, these technologies may also image losses of surface water to groundwater[57]. Another advantage is that they may provide information on structural controls on zones of focused GWSWE expressed at the surface. However, interpretation of EC patterns from these technologies is inherently uncertain due to the fact that (with the exception of specific conductance probes) the bulk EC of the sediments is measured. Variations in lithology (e.g., porosity, grain size distribution, which determine the strength of surface conduction) can be misinterpreted as variations in the ionic composition of groundwater.
Characterization Methods
Specific Conductance Probes
The simplest EC-based technology is a specific conductance probe, which measures the specific conductance of water between a small pair of metal plates at the end of the sensor probe. Many commercially available water quality sensors have a specific conductance sensor and a temperature sensor integrated into a single probe (they often also measure other water quality parameters, including pH and dissolved oxygen (DO) content). These are direct sensing measurements with a small footprint (the size of the sensor), so this is usually a time-consuming, inefficient method for detecting GWSWE dynamics. Furthermore, the sampling volume of the measurement is small (on the order of a cubic centimeter or less), so the degree to which the spot measurement is representative of larger-scale hydrological exchanges is often uncertain. However, specific conductance sensor remains popular, especially when integrated with a point temperature sensor, such as the Trident Probe.
Frequency Domain Electromagnetic (EM) Sensing Systems
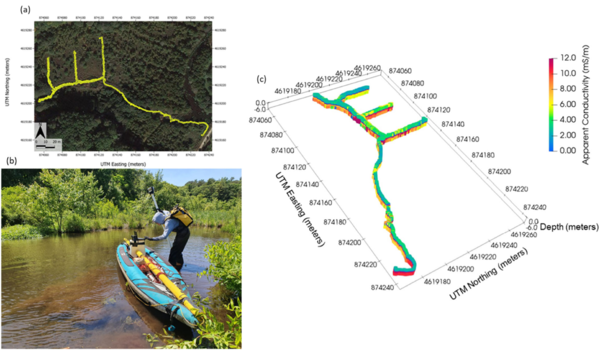
Electromagnetic (EM) sensors non-invasively sense the bulk EC of sediments (a function of both fluid composition and lithology as mentioned above) by measuring eddy currents induced in conductors using time varying electric and magnetic fields based on the physics of electromagnetic induction. Modern EM systems can simultaneously image across a range of depths. Frequency domain EM (FDEM) instruments generate a current that varies sinusoidally with time at a fixed frequency that is selected on the basis of desired exploration depth and resolution. State of the art FDEM sensors use a combination of different coil separations and/or frequencies to resolve conductivity structure over a range of depths. These instruments typically provide high-resolution (sub-meter) information on the EC structure in the upper 5 m (approximately, depending on EC) of the subsurface. Measurements are non-invasively and continuously made, meaning that large areas can be quickly surveyed on foot (e.g., along a shoreline) or from a boat in shallow water (1 m or less deep), for example when pulled along a river or stream channel. The method can also be deployed effectively in wetlands (Figure 5). FDEM data are often presented in terms of variations in the raw measurements because apparent EC values do not represent the true EC of the subsurface. However, with the increasing popularity of sensors with combinations of coil separations, the datasets can be inverted to obtain a model of the distribution of the true EC of the subsurface on land or below a water layer. Inversion of FDEM datasets is usually performed as a series of one-dimensional (1D) models, constrained to have a limited variance from each other, to generate a pseudo-2D model of the subsurface. Open-source software, such as EMagPy[58], is freely available to manage, visualize and interpret FDEM datasets.
Time Domain EM Sensing Systems
Time domain EM (TEM) systems transmit a current that is abruptly shut off (reduced to zero), resulting in a transient current flow that propagates (with decaying amplitude) into the earth. The time-decaying voltage recorded in a receiver coil contains information on the EC variation with depth below the instrument. TEM systems specifically designed for waterborne surveys provide investigation depths up to 70 m (again depending on electrical conductivity)[59]. Airborne TEM systems can also be deployed to look at large-scale surface water/groundwater dynamics, for example submarine discharge or saline intrusion along coastlines[60]. Inverse methods are employed to convert the raw measurements obtained along a transect into a distribution of conductivity.
Waterborne Electrical Imaging
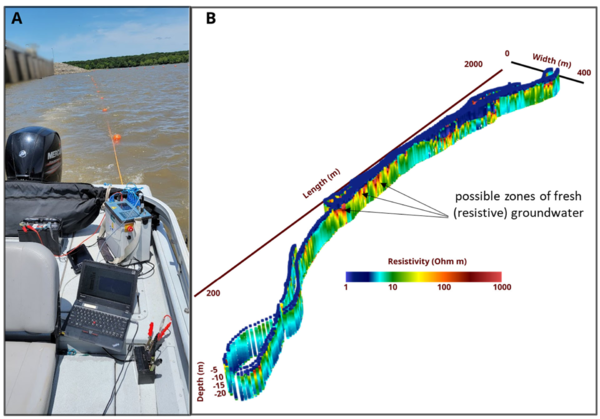
Electrical imaging techniques are based on galvanic (direct) contact between electrodes used to inject currents (and measure voltages) and the subsurface[56]. Relative to EM methods, this can be a disadvantage when surveying on land. However, when making measurements from a water body, the electrodes used to acquire the data can be deployed as a floating array that is pulled behind a vessel. Waterborne electrical imaging relies on acquiring measurements of electrical potential differences between different pairs of electrodes on the array while current is passed between one pair of electrodes[61]. As the array is pulled behind the boat, thousands of measurements are made along a survey transect. Similar to the EM methods, inverse methods are used to process these datasets and generate a 2D image of the variation in the conductivity of the sediments below the bed. Open-source software such as ResIPy support 2D or 3D inversion of waterborne datasets. Figure 6 shows results of a waterborne electrical imaging survey conducted to locate regions where relative fresh (electrically resistive) groundwater is discharging into the near shore environment in a coastal setting. Beneath the saline (low resistivity) water layer, spatial variability in resistivity may partly be related to variations in the pore-filling fluid conductivity, with localized resistive zones possibly indicating upwelling fresh groundwater. However, the variation in resistivity in the sediments below the water layer may reflect variations in lithology. An extension of the electrical imaging method involves collecting induced polarization (IP) data[56] in addition to electrical resistivity data. IP measurements capture the temporary charge storage characteristics of the subsurface, which are strongly controlled by lithology, with finer-grained (e.g. clay rich) sediments being more chargeable than coarser grained sediments. The method can be particularly useful for differentiating between conductivity variations resulting from variations in pore fluid specific conductance and those conductivity variations associated with lithology. For example, based on electrical imaging methods alone (or the EM method alone), it may not be possible to distinguish a zone of high specific conductance groundwater entering into freshwater from a region of relatively finer- grained sediments without additional supporting data (e.g. a core). IP measurements may be able to resolve this ambiguity as the region of finer-grained sediments will be more chargeable than the surrounding areas.
Monitoring Methods
Land-based Electrical Monitoring
There is increasing interest in the use of electrical imaging methods as monitoring systems. Semi-permanent arrays of electrodes can be installed to monitor groundwater/surface water dynamics over periods of days to years. Low-power instrumentation has been developed to specifically address the needs for long-term monitoring, although such instrumentation is not yet commercially available. Consequently, electrical monitoring of groundwater/surface water interactions currently remains in the realm of the research-driven specialist.
Considerations for Using EM and Waterborne Electrical Imaging Methods
The EM and waterborne electrical imaging methods both provide a way to determine variations in bulk electrical conductivity associated with groundwater/surface water interactions. However, each method has some advantages and some disadvantages. One consideration is maneuverability, particularly in shallow water environments. FDEM instruments are the most maneuverable, although they offer only limited investigation depths. Although bigger than the shallow-sensing frequency domain EM systems, TEM systems are still relatively maneuverable on water bodies. Whereas FDEM systems can be operated from a single small vessel, the TEM deployments require the use of pontoons as the transmitter and receiver coils need to be separated 9 m apart. This still equates to good maneuverability compared to waterborne electrical imaging where a floating electrode cable, typically 30-50 m long, is pulled behind a vessel.
In all three methods, variations in the water layer depth and the specific conductance of the water can significantly affect the data, especially in deeper water. Therefore, it is common to continuously record these parameters with an echo sounder and a specific conductance probe suspended in the water layer.
Other Hydrogeophysical Technologies
A number of other hydrogeophysical technologies exist, with proven applications to the characterization of settings where GWSWE occurs. Seismic reflection and refraction methods are used to image the depositional environments along coastlines. Ground penetrating radar has been effectively used to image depositional environments around freshwater lake shorelines, and across streams and rivers. Such information may help to identify depositional features that promote GWSWE but, unlike the temperature- and conductivity-based methods, do not sense changes in physical properties associated with the exchanging water itself.
One promising technique for detecting GWSWE is known as the self-potential (SP) method. This simple to deploy geophysical technique is based on mapping voltage differences caused by natural sources of electric current in the Earth that are generated through a number of coupled flow processes, one being the coupling of pore fluid flow and transport of electric charge. Zones of enhanced seepage within a porous medium can result in a significant ‘streaming potential’ due to charge transport induced by fluid flow. This phenomenon has been effectively used to locate zones of leakage through dams and embankments[62]. Recently, floating SP measurements have been used to define gaining and losing portions of streams and to identify evidence of focused exchange[63]. Although the data acquisition is simple, consisting of a pair of non-polarizing electrodes and a voltmeter, the interpretation of SP measurements requires expert knowledge to filter out confounding contributions to the recorded signals.
Guidelines for Implementing Hydrogeophysical Methods into Groundwater/Surface Water Interaction Studies
A number of factors will affect the success of individual hydrogeophysical methods at a specific site of GWSWE. Depending on site conditions and the objective, some methods may be inappropriate to deploy. For example, temperature-based methods will most likely succeed at times of the year and times of day when contrasts between upwelling groundwater and surface water are greatest. In contrast, it is quite possible that some sites of groundwater/surface water exchange will have an insufficient contrast in the specific conductance of the groundwater versus the surface water to make techniques based on EC measurements effective. A groundwater-surface water method selection tool (GW/SW-MST[64]) has recently been developed to assist practitioners in the informed selection of the methods that will be most effective for a particular site at a particular time. The tool guides the user through a series of questions that consider both the specific conditions at the site and the primary objectives of the investigation. The methods selection tool discusses the application of a number of additional technologies besides those included in this article. The selection tool is recommended as the starting point for any practitioner.
Summary
A number of temperature-based and electrical conductivity-based technologies exist for monitoring GWSWE over a range of spatial scales. Many of these technologies are most powerful when used as reconnaissance tools to rapidly identify probable locations of GWSWE to be verified with a limited campaign of direct sensing measurements (traditionally seepage meters). Vertical temperature profilers (VTPs) offer direct quantification of fluxes at sites identified by the reconnaissance tools, and some studies show that these methods are more reliable than traditional seepage meters. Given the number of sites across the globe where contaminated groundwater is impacting surface water resources, use of these technologies for both characterization and monitoring is expected to become more common.
References
- ^ 1.0 1.1 1.2 Lang, J.R., McDonough, J., Guillette, T.C., Storch, P., Anderson, J., Liles, D., Prigge, R., Miles, J.A.L., Divine, C., 2022. Characterization of per- and polyfluoroalkyl substances on fire suppression system piping and optimization of removal methods. Chemosphere, 308(Part 2), 136254. doi: 10.1016/j.chemosphere.2022.136254 Open Access Article
- ^ 2.0 2.1 2.2 Ross, I., and Storch, P., 2020. Foam Transition: Is It as Simple as "Foam Out / Foam In?". The Catalyst (Journal of JOIFF, The International Organization for Industrial Emergency Services Management), Q2 Supplement, 20 pages. Industry Newsletter
- ^ Kappetijn, K., 2023. Replacement of fluorinated extinguishing foam: When is clean clean enough? The Catalyst (Journal of JOIFF, The International Organization for Industrial Emergency Services Management), Q1 2023, pp. 31-33. Industry Newsletter
- ^ US Department of Defense, 2023. Performance Specification for Fire Extinguishing Agent, Fluorine-Free Foam (F3) Liquid Concentrate for Land-Based, Fresh Water Applications. Mil-Spec MIL-PRF-32725, 18 pages. Military Specification Document
- ^ Giesy, J.P., Kannan, K., 2001. Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environmental Science and Technology 35(7), pp. 1339-1342. doi: 10.1021/es001834k
- ^ Krafft, M.P., Riess, J.G., 2015. Selected physicochemical aspects of poly- and perfluoroalkylated substances relevant to performance, environment and sustainability-Part one. Chemosphere, 129, pp. 4-19. doi: 10.1016/j.chemosphere.2014.08.039
- ^ Higgins, C.P., Luthy, R.G., 2006. Sorption of Perfluorinated Surfactants on Sediments. Environmental Science and Technology, 40(23), pp. 7251-7256. doi: 10.1021/es061000n
- ^ Brusseau, M.L., 2018. Assessing the Potential Contributions of Additional Retention Processes to PFAS Retardation in the Subsurface. Science of the Total Environment, 613-614, pp. 176-185. doi: 10.1016/j.scitotenv.2017.09.065 Open Access Manuscript
- ^ Fukuda, H., Kawata, K., Okuda, H., 1990. Bilayer-Forming Ion-Pair Amphiphiles from Single-Chain Surfactants. Journal of the American Chemical Society, 112(4), pp. 1635-1637. doi: 10.1021/ja00160a057
- ^ 10.0 10.1 Krafft, M.P., 2006. Highly fluorinated compounds induce phase separation in, and nanostructuration of liquid media. Possible impact on, and use in chemical reactivity control. Journal of Polymer Science Part A: Polymer Chemistry, 44(14), pp. 4251-4258. doi: 10.1002/pola.21508 Open Access Article
- ^ Krafft, M.P., Guilieri, F., Riess, J.G., 1993. Can Single-Chain Perfluoroalkylated Amphiphiles Alone form Vesicles and Other Organized Supramolecular Systems? Angewandte Chemie International Edition in English, 32(5), pp. 741-743. doi: 10.1002/anie.199307411
- ^ 12.0 12.1 Krafft, M.P., Guilieri, F., Riess, J.G., 1994. Supramolecular assemblies from single chain perfluoroalkylated phosphorylated amphiphiles. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 84(1), pp. 113-119. doi: 10.1016/0927-7757(93)02681-4
- ^ 13.0 13.1 13.2 Krafft, M.P., Riess, J.G., 1998. Highly Fluorinated Amphiphiles and Collodial Systems, and their Applications in the Biomedical Field. A Contribution. Biochimie, 80(5-6), pp. 489-514. doi: 10.1016/S0300-9084(00)80016-4
- ^ Ringsdorf, H., Schlarb, B., Venzmer, J., 1988. Molecular Architecture and Function of Polymeric Oriented Systems: Models for the Study of Organization, Surface Recognition, and Dynamics of Biomembranes. Angewandte Chemie International Edition in English, 27(1), pp. 113-158. doi: 10.1002/anie.198801131
- ^ Israelachvili, J.N., Mitchell, D.J., Ninham, B.W., 1976. Theory of Self-Assembly of Hydrocarbon Amphiphiles into Micelles and Bilayers. Journal of the Chemical Society, Faraday Transactions 2: Molecular and Chemical Physics, 72, pp. 1525-1568. doi: 10.1039/F29767201525
- ^ Erkoç, Ş., Erkoç, F., 2001. Structural and electronic properties of PFOS and LiPFOS. Journal of Molecular Structure: THEOCHEM, 549(3), pp. 289-293. doi:10.1016/S0166-1280(01)00553-X
- ^ 17.0 17.1 Torres, F.J., Ochoa-Herrera, V., Blowers, P., Sierra-Alvarez, R., 2009. Ab initio study of the structural, electronic, and thermodynamic properties of linear perfluorooctane sulfonate (PFOS) and its branched isomers. Chemosphere 76(8), pp. 1143-1149. doi: 10.1016/j.chemosphere.2009.04.009
- ^ Abbandonato, G., Catalano, D., Marini, A., 2010. Aggregation of Perfluoroctanoate Salts Studied by 19F NMR and DFT Calculations: Counterion Complexation, Poly(ethylene glycol) Addition, and Conformational Effects. Langmuir 26(22), pp. 16762-16770. doi: 10.1021/la102578k.
- ^ Barton, S.W., Goudot, A., Bouloussa, O., Rondelez, F., Lin, B., Novak, F., Acero, A., Rice, S., 1992. Structural transitions in a monolayer of fluorinated amphiphile molecules. The Journal of Chemical Physics, 96(2), pp. 1343-1351. doi: 10.1063/1.462170
- ^ Barton, C.A., Butler, L.E., Zarzecki, C.J., Flaherty, J., Kaiser, M., 2006. Characterizing Perfluorooctanoate in Ambient Air near the Fence Line of a Manufacturing Facility: Comparing Modeled and Monitored Values. Journal of the Air and Waste Management Association, 56, pp. 48-55. doi: 10.1080/10473289.2006.10464429 Open Access Article
- ^ Krafft, M.P., Guilieri, F., Riess, J.G., 1993. Can Single-Chain Perfluoroalkylated Amphiphiles Alone form Vesicles and Other Organized Supramolecular Systems? Angewandte Chemie International Edition in English, 32(5), pp. 741-743. doi: 10.1002/anie.199307411
- ^ Furuya, H., Moroi, Y., Kaibara, K., 1996. Solid and Solution Properties of Alkylammonium Perfluorocarboxylates. The Journal of Physical Chemistry, 100(43), pp. 17249-17254. doi: 10.1021/jp9612801
- ^ Giulieri, F., Krafft, M.P., 1996. Self-organization of single-chain fluorinated amphiphiles with fluorinated alcohols. Thin Solid Films, 284-285, pp. 195-199. doi: 10.1016/S0040-6090(95)08304-9
- ^ Gladysz, J.A., Curran, D.P., Horvath, I.T., 2004. Handbook of Fluorous Chemistry. WILEY-VCH Verlag GmbH & Co. KGaA,, Weinheim, Germany. ISBN: 3-527-30617-X
- ^ Rojas, O.J., Macakova, L., Blomberg, E., Emmer, A., and Claesson, P.M., 2002. Fluorosurfactant Self-Assembly at Solid/Liquid Interfaces. Langmuir, 18(21), pp. 8085-8095. doi: 10.1021/la025989c
- ^ 26.0 26.1 Lombardo, D., Kiselev, M.A., Magazù, S., Calandra, P., 2015. Amphiphiles Self-Assembly: Basic Concepts and Future Perspectives of Supramolecular Approaches. Advances in Condensed Matter Physics, vol. 2015, article ID 151683, 22 pages. doi: 10.1155/2015/151683 Open Access Article
- ^ 27.0 27.1 Queensland (Australia) Department of Environment and Heritage Protection, 2016. Operational Policy - Environmental Management of Firefighting Foam. 16 pages. Free Download
- ^ U.S. Congress, 2019. S.1790 - National Defense Authorization Act for Fiscal Year 2020. United States Library of Congress. Text and History of Law.
- ^ Arizona State Legislature, 2019. Title 36, Section 1696. Firefighting foam; prohibited uses; exception; definitions. Text of Law
- ^ California Legislature, 2020. Senate Bill No. 1044, Chapter 308, Firefighting equipment and foam: PFAS chemicals. Text and History of Law
- ^ Arkansas General Assembly, 2021. An Act Concerning the Use of Certain Chemicals in Firefighting Foam; and for Other Purposes. Act 315, State of Arkansas. Text and History of Law.
- ^ Espinosa, Summers, Kelly, J., Statler, Hansen, Young, 2021. Amendment to Fire Prevention and Control Act. House Bill 2722. West Virginia Legislature. Text and History of Law
- ^ Louisiana Legislature, 2021. Act No. 232. Text and History of Law
- ^ Vermont Legislature, 2021b. Act No. 36, PFAS in Class B Firefighting Foam. History and Text of Law
- ^ U.S. Congress, 2021. S.2792 - National Defense Authorization Act for Fiscal Year 2021. United States Library of Congress. Text and History of Law.
- ^ National Fire Protection Association (NFPA), 2022. Codes and Standards, 409: Standard on Aircraft Hangars. NFPA Website
- ^ Constantz, J., 2008. Heat as a Tracer to Determine Streambed Water Exchanges. Water Resources Research, 44 (4).doi: 10.1029/2008WR006996.Open Access Article
- ^ Briggs, M. A., Jackson, K. E., Liu, F., Moore, E. M., Bisson, A., Helton, A. M., 2022. Exploring Local Riverbank Sediment Controls on the Occurrence of Preferential Groundwater Discharge Points. Water, 14(1). doi: 10.3390/w14010011 Open Access Article.
- ^ Briggs, M. A., Wang, C., Day-Lewis, F. D., Williams, K. H., Dong, W., Lane, J. W., 2019. Return Flows from Beaver Ponds Enhance Floodplain-to-River Metals Exchange in Alluvial Mountain Catchments. Science of the Total Environment, 685, pp. 357–369. doi: 10.1016/j.scitotenv.2019.05.371. Open Access Manuscript
- ^ Mangel, A. R., Dawson, C. B., Rey, D. M., Briggs, M. A., 2022. Drone Applications in Hydrogeophysics: Recent Examples and a Vision for the Future. The Leading Edge, 41 (8), pp. 540–547. doi: 10.1190/tle41080540.
- ^ Mwakanyamale, K., Slater, L., Day-Lewis, F., Elwaseif, M., Johnson, C., 2012. Spatially Variable Stage-Driven Groundwater-Surface Water Interaction Inferred from Time-Frequency Analysis of Distributed Temperature Sensing Data. Geophysical Research Letters, 39(6). doi: 10.1029/2011GL050824. Open Access Article
- ^ 42.0 42.1 Slater, L. D., Ntarlagiannis, D., Day-Lewis, F. D., Mwakanyamale, K., Versteeg, R. J., Ward, A., Strickland, C., Johnson, C. D., Lane Jr., J. W., 2010. Use of Electrical Imaging and Distributed Temperature Sensing Methods to Characterize Surface Water-Groundwater Exchange Regulating Uranium Transport at the Hanford 300 Area, Washington. Water Resources Research, 46(10). doi: 10.1029/2010WR009110. Open Access Article
- ^ 43.0 43.1 Selker, J. S., Thévenaz, L., Huwald, H., Mallet, A., Luxemburg, W., van de Giesen, N., Stejskal, M., Zeman, J., Westhoff, M., Parlange, M. B., 2006. Distributed Fiber-Optic Temperature Sensing for Hydrologic Systems. Water Resources Research, 42 (12). doi: 10.1029/2006WR005326. Open Access Article
- ^ 44.0 44.1 Tyler, S. W., Selker, J. S., Hausner, M. B., Hatch, C. E., Torgersen, T., Thodal, C. E., Schladow, S. G., 2009. Environmental Temperature Sensing Using Raman Spectra DTS Fiber-Optic Methods. Water Resources Research, 45(4). doi: 10.1029/2008WR007052. Open Access Article
- ^ Hare, D. K., Briggs, M. A., Rosenberry, D. O., Boutt, D. F., Lane Jr., J. W., 2015. A Comparison of Thermal Infrared to Fiber-Optic Distributed Temperature Sensing for Evaluation of Groundwater Discharge to Surface Water. Journal of Hydrology, 530, pp. 153–166. doi: 10.1016/j.jhydrol.2015.09.059.
- ^ 46.0 46.1 Irvine, D. J., Briggs, M. A., Cartwright, I., Scruggs, C. R., Lautz, L. K., 2016. Improved Vertical Streambed Flux Estimation Using Multiple Diurnal Temperature Methods in Series. Groundwater, 55(1), pp. 73-80. doi: 10.1111/gwat.12436.
- ^ Koch, F. W., Voytek, E. B., Day-Lewis, F. D., Healy, R., Briggs, M. A., Lane Jr., J. W., Werkema, D., 2016. 1DTempPro V2: New Features for Inferring Groundwater/Surface-Water Exchange. Groundwater, 54(3), pp. 434–439. doi: 10.1111/gwat.12369.
- ^ 48.0 48.1 48.2 48.3 Irvine, D. J., Briggs, M. A., Lautz, L. K., Gordon, R. P., McKenzie, J. M., Cartwright, I., 2017. Using Diurnal Temperature Signals to Infer Vertical Groundwater-Surface Water Exchange. Groundwater, 55(1), pp. 10–26. doi: 10.1111/gwat.12459. Open Access Manuscript
- ^ Briggs, M. A., Lautz, L. K., Buckley, S. F., Lane Jr., J. W., 2014. Practical Limitations on the Use of Diurnal Temperature Signals to Quantify Groundwater Upwelling. Journal of Hydrology, 519(B), pp. 1739–1751. doi: 10.1016/j.jhydrol.2014.09.030.
- ^ 50.0 50.1 Gordon, R. P., Lautz, L. K., Briggs, M. A., McKenzie, J. M., 2012. Automated Calculation of Vertical Pore-Water Flux from Field Temperature Time Series Using the VFLUX Method and Computer Program. Journal of Hydrology, 420–421, pp. 142–158. doi: 10.1016/j.jhydrol.2011.11.053.
- ^ 51.0 51.1 Irvine, D. J., Lautz, L. K., Briggs, M. A., Gordon, R. P., McKenzie, J. M., 2015. Experimental Evaluation of the Applicability of Phase, Amplitude, and Combined Methods to Determine Water Flux and Thermal Diffusivity from Temperature Time Series Using VFLUX 2. Journal of Hydrology, 531(3), pp. 728–737. doi: 10.1016/j.jhydrol.2015.10.054.
- ^ Sohn, R. A., Harris, R. N., 2021. Spectral Analysis of Vertical Temperature Profile Time-Series Data in Yellowstone Lake Sediments. Water Resources Research, 57(4), e2020WR028430. doi: 10.1029/2020WR028430. Open Access Article
- ^ Koch, F. W., Voytek, E. B., Day-Lewis, F. D., Healy, R., Briggs, M. A., Werkema, D., Lane Jr., J. W., 2015. 1DTempPro: A Program for Analysis of Vertical One-Dimensional (1D) Temperature Profiles v2.0. U.S. Geological Survey Software Release. doi: 10.5066/F76T0JQS. Free Download from USGS
- ^ 54.0 54.1 54.2 McAliley, W. A., Day-Lewis, F. D., Rey, D., Briggs, M. A., Shapiro, A. M., Werkema, D., 2022. Application of Recursive Estimation to Heat Tracing for Groundwater/Surface-Water Exchange. Water Resources Research, 58(6), e2021WR030443. doi: 10.1029/2021WR030443. Open Access Article
- ^ Cox, M. H., Su, G. W., Constantz, J., 2007. Heat, Chloride, and Specific Conductance as Ground Water Tracers near Streams. Groundwater, 45(2), pp. 187–195. doi: 10.1111/j.1745-6584.2006.00276.x.
- ^ 56.0 56.1 56.2 Binley, A., Slater, L., 2020. Resistivity and Induced Polarization: Theory and Applications to the Near-Surface Earth. Cambridge University Press. doi: 10.1017/9781108685955.
- ^ Johnson, T. C., Slater, L. D., Ntarlagiannis, D., Day-Lewis, F. D., Elwaseif, M., 2012. Monitoring Groundwater-Surface Water Interaction Using Time-Series and Time- Frequency Analysis of Transient Three-Dimensional Electrical Resistivity Changes. Water Resources Research, 48(7). doi: 10.1029/2012WR011893. Open Access Article
- ^ McLachlan, P., Blanchy, G., Binley, A., 2021. EMagPy: Open-Source Standalone Software for Processing, Forward Modeling and Inversion of Electromagnetic Induction Data. Computers and Geosciences, 146, 104561. doi: 10.1016/j.cageo.2020.104561.
- ^ Lane Jr., J. W., Briggs, M. A., Maurya, P. K., White, E. A., Pedersen, J. B., Auken, E., Terry, N., Minsley, B., Kress, W., LeBlanc, D. R., Adams, R., Johnson, C. D., 2020. Characterizing the Diverse Hydrogeology Underlying Rivers and Estuaries Using New Floating Transient Electromagnetic Methodology. Science of the Total Environment, 740, 140074. doi: 10.1016/j.scitotenv.2020.140074. Open Access Manuscript
- ^ d’Ozouville, N., Auken, E., Sorensen, K., Violette, S., de Marsily, G., Deffontaines, B., Merlen, G., 2008. Extensive Perched Aquifer and Structural Implications Revealed by 3D Resistivity Mapping in a Galapagos Volcano. Earth and Planetary Science Letters, 269(3–4), pp. 518–522. doi: 10.1016/j.epsl.2008.03.011.
- ^ Day-Lewis, F. D., White, E. A., Johnson, C. D., Lane Jr, J. W., Belaval, M., 2006. Continuous Resistivity Profiling to Delineate Submarine Groundwater Discharge—Examples and Limitations. The Leading Edge, 25(6), pp. 724–728. doi: 10.1190/1.2210056
- ^ Panthulu, T. V, Krishnaiah, C., Shirke, J. M., 2001. Detection of Seepage Paths in Earth Dams Using Self-Potential and Electrical Resistivity Methods. Engineering Geology, 59(3-4), pp. 281–295. doi: 10.1016/S0013-7952(00)00082-X.
- ^ Ikard, S. J., Teeple, A. P., Payne, J. D., Stanton, G. P., Banta, J. R., 2018. New Insights On Scale-Dependent Surface-Groundwater Exchange from a Floating Self-Potential Dipole. Journal of Environmental and Engineering Geophysics, 23(2), pp. 261–287. doi: 10.2113/JEEG23.2.261.
- ^ Hammett, S., Day-Lewis, F. D., Trottier, B., Barlow, P. M., Briggs, M. A., Delin, G., Harvey, J. W., Johnson, C. D., Lane jr., J. W., Rosenberry, D. O., Werkema, D. D., 2022. GW/SW-MST: A Groundwater/Surface-Water Method Selection Tool. Groundwater, 60(6), pp. 784-791. doi: 10.1111/gwat.13194. Open Access Manuscript
See Also
USGS Water Resources: