|
|
| (376 intermediate revisions by the same user not shown) |
| Line 1: |
Line 1: |
| − | ==Contaminated Sediment Risk Assessment== | + | ==Photoactivated Reductive Defluorination PFAS Destruction== |
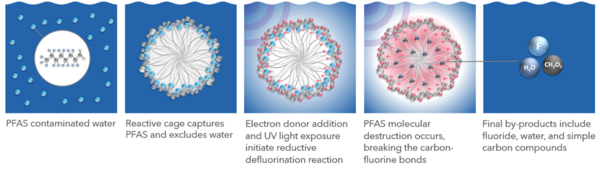
| − | [[Contaminated Sediments - Introduction | Contaminated sediments]] in rivers and streams, lakes, coastal harbors, and estuaries have the potential to pose ecological and human health risks. The goals of risk assessment applied to contaminated sediments are to characterize the nature and magnitude of the current and potential threats to human health, wildlife and ecosystem functioning posed by contamination; identify the key factors contributing to the potential health and ecological risks; evaluate how implementation of one or more remedy actions will mitigate the risks in the short and long term; and evaluate the risks and impacts from sediment management, both during and after any dredging or other remedy construction activities. | + | Photoactivated Reductive Defluorination (PRD) is a [[Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) | PFAS]] destruction technology predicated on [[Wikipedia: Ultraviolet | ultraviolet (UV)]] light-activated photochemical reactions. The destruction efficiency of this process is enhanced by the use of a [[Wikipedia: Surfactant | surfactant]] to confine PFAS molecules in self-assembled [[Wikipedia: Micelle | micelles]]. The photochemical reaction produces [[Wikipedia: Solvated electron | hydrated electrons]] from an electron donor that associates with the micelle. The hydrated electrons have sufficient energy to rapidly cleave fluorine-carbon and other molecular bonds of PFAS molecules due to the association of the electron donor with the micelle. Micelle-accelerated PRD is a highly efficient method to destroy PFAS in a wide variety of water matrices. |
| | <div style="float:right;margin:0 0 2em 2em;">__TOC__</div> | | <div style="float:right;margin:0 0 2em 2em;">__TOC__</div> |
| | | | |
| | '''Related Article(s):''' | | '''Related Article(s):''' |
| − | *[[Contaminated Sediments - Introduction]] | + | *[[Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS)]] |
| − | *[[In Situ Treatment of Contaminated Sediments with Activated Carbon]] | + | *[[PFAS Sources]] |
| − | *[[Sediment Capping]] | + | *[[PFAS Transport and Fate]] |
| − | *[[Passive Sampling of Sediments]] | + | *[[PFAS Ex Situ Water Treatment]] |
| | + | *[[Supercritical Water Oxidation (SCWO)]] |
| | + | *[[PFAS Treatment by Electrical Discharge Plasma]] |
| | | | |
| | '''Contributor(s):''' | | '''Contributor(s):''' |
| − | *Richard J. Wenning | + | *Dr. Suzanne Witt |
| − | *Sabine E. Apitz | + | *Dr. Meng Wang |
| | + | *Dr. Denise Kay |
| | | | |
| | '''Key Resource(s):''' | | '''Key Resource(s):''' |
| − | * Contaminated Sediment Remediation Guidance for Hazardous Waste Sites<ref name="USEPA2005">United States Environmental Protection Agency (USEPA), 2005. Contaminated Sediment Remediation Guidance for Hazardous Waste Sites. Office of Solid Waste and Emergency Response, Washington, D.C. EPA-540-R-05-012. OSWER 9355.0-85. Free download from: [https://semspub.epa.gov/work/HQ/174471.pdf USEPA] [[Media: EPA-540-R-05-012.pdf | Report.pdf]]</ref> | + | *Efficient Reductive Destruction of Perfluoroalkyl Substances under Self-Assembled Micelle Confinement<ref name="ChenEtAl2020">Chen, Z., Li, C., Gao, J., Dong, H., Chen, Y., Wu, B., Gu, C., 2020. Efficient Reductive Destruction of Perfluoroalkyl Substances under Self-Assembled Micelle Confinement. Environmental Science and Technology, 54(8), pp. 5178–5185. [https://doi.org/10.1021/acs.est.9b06599 doi: 10.1021/acs.est.9b06599]</ref> |
| − | | + | *Complete Defluorination of Perfluorinated Compounds by Hydrated Electrons Generated from 3-Indole-Acetic-Acid in Organomodified Montmorillonite<ref name="TianEtAl2016">Tian, H., Gao, J., Li, H., Boyd, S.A., Gu, C., 2016. Complete Defluorination of Perfluorinated Compounds by Hydrated Electrons Generated from 3-Indole-Acetic-Acid in Organomodified Montmorillonite. Scientific Reports, 6(1), Article 32949. [https://doi.org/10.1038/srep32949 doi: 10.1038/srep32949] [[Media: TianEtAl2016.pdf | Open Access Article]]</ref> |
| − | * Principles for Environmental Risk Assessment of the Sediment Compartment<ref name="Tarazona2014">Tarazona, J.V., Versonnen, B., Janssen, C., De Laender, F., Vangheluwe, M. and Knight, D., 2014. Principles for Environmental Risk Assessment of the Sediment Compartment: Proceedings of the Topical Scientific Workshop. 7-8 May 2013. European Chemicals Agency, Helsinki. Document ECHA-14-R-13-EN. Free download from: [https://echa.europa.eu/documents/10162/22816050/environmental_risk_assessment_final_en.pdf/3515b685-6601-40ce-bd48-3f8d5332c0f8 European Chemicals Agency] [[Media: ECHA-14-R-13-EN.pdf | Report.pdf]]</ref> | + | *Application of Surfactant Modified Montmorillonite with Different Conformation for Photo-Treatment of Perfluorooctanoic Acid by Hydrated Electrons<ref name="ChenEtAl2019">Chen, Z., Tian, H., Li, H., Li, J. S., Hong, R., Sheng, F., Wang, C., Gu, C., 2019. Application of Surfactant Modified Montmorillonite with Different Conformation for Photo-Treatment of Perfluorooctanoic Acid by Hydrated Electrons. Chemosphere, 235, pp. 1180–1188. [https://doi.org/10.1016/j.chemosphere.2019.07.032 doi: 10.1016/j.chemosphere.2019.07.032]</ref> |
| − | | + | *[https://serdp-estcp.mil/projects/details/c4e21fa2-c7e2-4699-83a9-3427dd484a1a ER21-7569: Photoactivated Reductive Defluorination PFAS Destruction]<ref name="WittEtAl2023">Kay, D., Witt, S., Wang, M., 2023. Photoactivated Reductive Defluorination PFAS Destruction: Final Report. ESTCP Project ER21-7569. [https://serdp-estcp.mil/projects/details/c4e21fa2-c7e2-4699-83a9-3427dd484a1a Project Website] [[Media: ER21-7569_Final_Report.pdf | Final Report.pdf]]</ref> |
| − | * Assessing and managing contaminated sediments: | |
| − | :: Part I, Developing an Effective Investigation and Risk Evaluation Strategy<ref name="Apitz2005a">Apitz, S.E., Davis, J.W., Finkelstein, K., Hohreiter, D.W., Hoke, R., Jensen, R.H., Jersak, J., Kirtay, V.J., Mack, E.E., Magar, V.S. and Moore, D., 2005. Assessing and Managing Contaminated Sediments: Part I, Developing an Effective Investigation and Risk Evaluation Strategy. Integrated Environmental Assessment and Management, 1(1), pp. 2-8. [https://doi.org/10.1897/IEAM_2004a-002.1 DOI: 10.1897/IEAM_2004a-002.1] Free access article from: [https://setac.onlinelibrary.wiley.com/doi/epdf/10.1897/IEAM_2004a-002.1 Society of Environmental Toxicology and Chemistry] [[Media: Apitz2005a.pdf | Report.pdf]]</ref>
| |
| − | :: Part II, Evaluating Risk and Monitoring Sediment Remedy Effectiveness<ref name="Apitz2005b">Apitz, S.E., Davis, J.W., Finkelstein, K., Hohreiter, D.W., Hoke, R., Jensen, R.H., Jersak, J., Kirtay, V.J., Mack, E.E., Magar, V.S. and Moore, D., 2005b. Assessing and Managing Contaminated Sediments: Part II, Evaluating Risk and Monitoring Sediment Remedy Effectiveness. Integrated Environmental Assessment and Management, 1(1), pp.e1-e14. [https://doi.org/10.1897/IEAM_2004a-002e.1 DOI: 10.1897/IEAM_2004a-002e.1]</ref>
| |
| | | | |
| | ==Introduction== | | ==Introduction== |
| − | Improving the management of [[Contaminated Sediments - Introduction | contaminated sediments]] is of growing concern globally. Sediment processes in both marine and freshwater environments are important to the function of aquatic ecosystems<ref name="Apitz2012">Apitz, S.E., 2012. Conceptualizing the role of sediment in sustaining ecosystem services: Sediment-Ecosystem Regional Assessment (SEcoRA), Science of the Total Environment, 415, pp. 9-30. [https://doi.org/10.1016/j.scitotenv.2011.05.060 DOI:10.1016/j.scitotenv.2011.05.060] Free download from: [https://d1wqtxts1xzle7.cloudfront.net/7588577/Apitz_SEcoRA%202012.pdf?1326618388=&response-content-disposition=inline%3B+filename%3DConceptualizing_the_role_of_sediment_in.pdf&Expires=1637094311&Signature=c2wczG59XxkitPjmBhc9PaODHJ8Vufg3gyzdG8tqGD6~mIVhLoz30E7eQNIghfMlH~jbch3KTVxMqD2AQFMQCSeXghIwqH~lXjGrEP07MJXCEgntzSW-V8Gws~33it5pEm9Ied64fSOvMLJR-PUXVr2OVTsVHQJHurHdGrtEmhUd90bKrC0NNlD28YLGQpkVUOlqa75e0K4sjPngwPUwUxhq18NAH6-1Uc3fQU5g5AjXwGph-VNe7EwzT-0do5OD056AsG-Eg8xIZi0ABJqMsg1wb92tIPpmmNy6ntdklHeN6tq~3IJFB7Tg8XYntQ-CGT8pYV9S7Kz14GhXVm9OQA__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA Academia.edu]</ref>, and many organisms rely on certain sediment quality and quantity characteristics for their life cycle<ref name="Hauer2018">Hauer, C., Leitner, P., Unfer, G., Pulg, U., Habersack, H. and Graf, W., 2018. The Role of Sediment and Sediment Dynamics in the Aquatic Environment. In: Schmutz S., Sendzimir J. (ed.s) Riverine Ecosystem Management. Aquatic Ecology Series, vol. 8, pp. 151-169. Springer. [https://doi.org/10.1007/978-3-319-73250-3_8 DOI: 10.1007/978-3-319-73250-3_8] Open access book from: [https://library.oapen.org/bitstream/handle/20.500.12657/27726/1002280.pdf?seque#page=153 SpringerOpen]</ref>. Human health can also be affected by sediment conditions, either via direct contact, as a result of sediment impacts on water quality, or because of the strong influence sediments can have on the quality of fish and shellfish consumed by people<ref name="Greenfield2015">Greenfield, B.K., Melwani, A.R. and Bay, S.M., 2015. A Tiered Assessment Framework to Evaluate Human Health Risk of Contaminated Sediment. Integrated Environmental Assessment and Management, 11(3), pp. 459-473. [https://doi.org/10.1002/ieam.1610 DOI: 10.1002/ieam.1610]</ref>. A common approach to achieving the explicit management goals inherent in different sediment assessment frameworks in North America and elsewhere is the use of the ecological risk assessment (ERA)<ref name="USEPA1997a">US Environmental Protection Agency (USEPA), 1997. The Incidence and Severity of Sediment Contamination in Surface Waters of the United States: Volume 1, National Sediment Quality Survey. EPA-823R-97-006. Washington, DC. [[Media: EPA-823-R-97-006.pdf | Report.pdf]]</ref>. An ERA “evaluates the likelihood and magnitude of adverse effects from exposure to a chemical for organisms, such as animals, plants, or microbes, in the environment”<ref name="SETAC2018">Society of Environmental Toxicology and Chemistry (SETAC), 2018. Technical Issue Paper: Environmental Risk Assessment of Chemicals. SETAC, Pensacola, FL. 5 pp. Free download from: [https://cdn.ymaws.com/www.setac.org/resource/resmgr/publications_and_resources/setac_tip_era.pdf SETAC] [[Media: setac_tip_era2018.pdf | Report.pdf]]</ref>. An ERA provides information relevant to the management decision-making process<ref name="Stahl2001">Stahl, R.G., Bachman, R., Barton, A., Clark, J., deFur, P., Ells, S., Pittinger, C., Slimak, M., Wentsel, R., 2001. Risk Management: Ecological Risk-Based Decision Making. SETAC Press, Pensacola, FL, 222 pp. ISBN: 978-1-880611-26-5</ref>. It should be performed in a scientifically based, defensible manner that is cost-effective and protective of human health and the environment<ref name="CNO1999">Chief of Naval Operations (CNO), 1999. Navy Policy for Conducting Ecological Risk Assessments, Letter 5090, Ser N453E/9U595355, dated 05 April 99. Department of the Navy, Washington, DC. Free download from: [https://www.navfac.navy.mil/content/dam/navfac/Specialty%20Centers/Engineering%20and%20Expeditionary%20Warfare%20Center/Environmental/Restoration/er_pdfs/gpr/cno-ev-pol-era-19990405.pdf the US Navy] [[Media: CNO1999.pdf | Report.pdf]]</ref>. Therefore, science-based methods for assessing sediment quality and use of risk-based decision-making in sediment management are important for identifying conditions suspected to adversely affect ecological and human services provided by sediments, and predicting the likely consequences of different sediment management actions<ref name="Bridges2006">Bridges, T.S., Apitz, S.E., Evison, L., Keckler, K., Logan, M., Nadeau, S. and Wenning, R.J., 2006. Risk‐Based Decision Making to Manage Contaminated Sediments. Integrated Environmental Assessment and Management, 2(1), pp. 51-58. [https://doi.org/10.1002/ieam.5630020110 DOI: 10.1002/ieam.5630020110] Free access article from: [https://setac.onlinelibrary.wiley.com/doi/epdf/10.1002/ieam.5630020110 SETAC]</ref><ref name="Apitz2011">Apitz, S.E., 2011. Integrated Risk Assessments for the Management of Contaminated Sediments in Estuaries and Coastal Systems. In: Wolanski, E. and McLusky, D.S. (eds.) Treatise on Estuarine and Coastal Science, Vol 4, pp. 311–338. Waltham: Academic Press. ISBN: 9780123747112</ref>.
| + | [[File:WittFig1.png | thumb |600px|Figure 1. Schematic of PRD mechanism<ref name="WittEtAl2023"/>]] |
| − | | + | The Photoactivated Reductive Defluorination (PRD) process is based on a patented chemical reaction that breaks fluorine-carbon bonds and disassembles PFAS molecules in a linear fashion beginning with the [[Wikipedia: Hydrophile | hydrophilic]] functional groups and proceeding through shorter molecules to complete mineralization. Figure 1 shows how PRD is facilitated by adding [[Wikipedia: Cetrimonium bromide | cetyltrimethylammonium bromide (CTAB)]] to form a surfactant micelle cage that traps PFAS. A non-toxic proprietary chemical is added to solution to associate with the micelle surface and produce hydrated electrons via stimulation with UV light. These highly reactive hydrated electrons have the energy required to cleave fluorine-carbon and other molecular bonds resulting in the final products of fluoride, water, and simple carbon molecules (e.g., formic acid and acetic acid). The methods, mechanisms, theory, and reactions described herein have been published in peer reviewed literature<ref name="ChenEtAl2020"/><ref name="TianEtAl2016"/><ref name="ChenEtAl2019"/><ref name="WittEtAl2023"/>. |
| − | Sediment risk assessment is increasingly used by governmental agencies to support sediment management in freshwater, estuarine, and marine environments. Strategies for sediment management encompass a wide variety of actions, from removal, capping or treatment of contaminated sediment to the monitoring of natural processes, including sedimentation, binding, and bio- and photo-degradation that serve to reduce the potential threat to aquatic life over time. It is not uncommon to revisit a sediment risk assessment periodically to check how changed environmental conditions reflected in sediment and biotic sampling work has either reduced or exacerbated the threats identified in the initial assessment.
| |
| − | | |
| − | At present, several countries lack common recommendations specific to conducting risk assessment of contaminated sediments<ref name="Bruce2020">Bruce, P., Sobek, A., Ohlsson, Y. and Bradshaw, C., 2020. Risk assessments of contaminated sediments from the perspective of weight of evidence strategies – a Swedish case study. Human and Ecological Risk Assessment, 27(5), pp. 1366-1387. [https://doi.org/10.1080/10807039.2020.1848414 DOI: 10.1080/10807039.2020.1848414] [https://www.tandfonline.com/doi/full/10.1080/10807039.2020.1848414 Website]</ref>. In the European Union, sediment has played a secondary role in the Water Framework Directive (WFD), with most quality standards being focused on water with the option for the development of national standards for sediment and biota for bioaccumulative compounds. The Common Implementation Strategy (CIS) in 2010 provided guidance on the monitoring of contaminants in sediments and biota, but not on risk-based decision-making<ref name="EC2010">European Commission, 2010. Common Implementation Strategy For The Water Framework Directive (2000/60/EC), Technical Report - 2010 – 041; Guidance document No. 25 On Chemical Monitoring Of Sediment And Biota Under The Water Framework Directive. 82pp. ISBN 978-92-79-16224-4. [https://op.europa.eu/en/publication-detail/-/publication/5ff7a8ec-995b-4d90-a140-0cc9b4bf980d Free download]</ref>. There are efforts underway to incorporate guidance for management of contaminated sediment in the Common Implementation Strategy in 2021<ref name="Brils2020">Brils, J., 2020. Including sediment in European River Basin Management Plans: Twenty years of work by SedNet. Journal of Soils and Sediments, 20(12), pp.4229-4237. [https://doi.org/10.1007/s11368-020-02782-1 DOI: 10.1007/s11368-020-02782-1] [https://link.springer.com/content/pdf/10.1007/s11368-020-02782-1.pdf Open Access Article]</ref>. Sediment risk assessment guidance from Norway, Canada, the Netherlands, and the US are most often referenced when assessing the risks from contaminated sediments<ref name="Bruce2020"/><ref name="Birch2018">Birch, G.F., 2018. A review of chemical-based sediment quality assessment methodologies for the marine environment. Marine Pollution Bulletin, 133, pp.218-232. [https://doi.org/10.1016/j.marpolbul.2018.05.039 DOI: 10.1016/j.marpolbul.2018.05.039]</ref><ref name="Kwok2014">Kwok, K.W., Batley, G.E., Wenning, R.J., Zhu, L., Vangheluwe, M. and Lee, S., 2014. Sediment quality guidelines: challenges and opportunities for improving sediment management. Environmental Science and Pollution Research, 21(1), pp. 17-27. [https://doi.org/10.1007/s11356-013-1778-7 DOI: 10.1007/s11356-013-1778-7] Free download from: [https://www.researchgate.net/profile/Graeme-Batley/publication/236836992_Sediment_quality_guidelines_Challenges_and_opportunities_for_improving_sediment_management/links/0c96052b8a8f5ad0c6000000/Sediment-quality-guidelines-Challenges-and-opportunities-for-improving-sediment-management.pdf ResearchGate]</ref>. Some European countries, such as Norway, have focused their risk assessment guidance on the assessment of sediment conditions relative to general chemical thresholds, while in North America, risk assessment guidance focuses on site- or region-specific conditions<ref name="Apitz2008">Apitz, S.E., 2008. Is risk-based, sustainable sediment management consistent with European policy?. Journal of Soils and Sediments, 8(6), p.461-466. [https://doi.org/10.1007/s11368-008-0039-8 DOI: 10.1007/s11368-008-0039-8] Free download from: [https://d1wqtxts1xzle7.cloudfront.net/7081664/apitz%20jss%20risk-based%20europe-with-cover-page-v2.pdf?Expires=1637274548&Signature=KqIoYyQ6VPAFN7lKHJMVC3bbn00RRMCR68bsQNBGrFJ9kbX5BnI-aucFCqRgVUNUb1lu0Q4tzUkCjPXJRGBsTA3OnbH8Ol9sNoXZ001aOwG7tKuV8qEblGiqtQUHh9GdiNAPQsm50f~E1iozL9a6imApWjqK8oFCfdUbcUd1oaW7PCDu28KWN-k5ddefWNZBAzGIdaWt3mBJ1EYeKRrp4F6Codlny3pWCT5MpA~c4c0IKq8L7Uj~-VxH5LXjFDd7cm07JeOY8S5rlxgF1zMoTIggMo5v2M3AS3CO2SAqy7yR3HC-IjUx3RsMqKa5eS2jT1ADiXcqeVygCdCCXza05g__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA Academia.edu]</ref>.
| |
| − | | |
| − | There is general consensus from a regulatory perspective, globally, on the importance of sediment risk assessment. Technical guidance documents prepared by Canada<ref name="Fletcher2008">Fletcher, R., Welsh, P. and Fletcher, T., 2008. Guidelines for Identifying, Assessing, and Managing Contaminated Sediments in Ontario. Ontario Ministry of the Environment. PIBS6658e. [http://www.ene.gov.on.ca/publications/6658e Website]</ref><ref name="HealthCanada2017">Health Canada, 2017. Supplemental Guidance on Human Health Risk Assessment of Contaminated Sediments: Direct Contact Pathway, Federal Contaminated Site Risk Assessment in Canada. ISBN: 978-0-660-07989-9. Cat.: H144-41/2017E-PDF. Pub. 160382. Free download from: [https://publications.gc.ca/collections/collection_2018/sc-hc/H144-41-2017-eng.pdf Health Canada] [[Media: HealthCanada2117.pdf | Report.pdf]]</ref> , the European Union<ref name="Tarazona2014"/>, and the United States Environmental Protection Agency (USEPA)<ref name="USEPA2005"/> advise a flexible, tiered approach for sediment risk assessment. Sediment quality guidelines in many countries reflect the scientific importance of including certain sediment-specific measurement and biotic assessment endpoints, as well as certain physical sediment processes and chemical transformation processes potentially affecting biotic responses to contaminant exposure in the sediment<ref name="Wenning2005">Wenning, R.J. Batley, G.E., Ingersoll, C.G., and Moore, D.W., (eds), 2005. Use Of Sediment Quality Guidelines And Related Tools For The Assessment Of Contaminated Sediments. SETAC, Pensacola, FL. 815 pp. ISBN 1-880611-71-6.</ref>. New risk assessment methods continue to emerge in the scientific literature<ref name="Benson2018">Benson, N.U., Adedapo, A.E., Fred-Ahmadu, O.H., Williams, A.B., Udosen, E.D., Ayejuyo, O.O. and Olajire, A.A., 2018. A new method for assessment of sediment-associated contamination risks using multivariate statistical approach. MethodsX, 5, pp. 268-276. [https://doi.org/10.1016/j.mex.2018.03.005 DOI: 10.1016/j.mex.2018.03.005] [https://www.sciencedirect.com/science/article/pii/S2215016118300438/pdfft?md5=85b8a3a1062310e4c7c4a06e670e66c4&pid=1-s2.0-S2215016118300438-main.pdf Free Access Article] [[Media: Benson2018.pdf | Report.pdf]]</ref><ref name="Saeedi2015">Saeedi, M. and Jamshidi-Zanjani, A., 2015. Development of a new aggregative index to assess potential effect of metals pollution in aquatic sediments. Ecological Indicators, 58, pp. 235-243. [https://doi.org/10.1016/j.ecolind.2015.05.047 DOI: 10.1016/j.ecolind.2015.05.047] Free download from: [https://www.academia.edu/download/49801572/mRAC_published.pdf Academis.edu]</ref><ref name="Vaananen2018">Väänänen, K., Leppänen, M.T., Chen, X. and Akkanen, J., 2018. Metal bioavailability in ecological risk assessment of freshwater ecosystems: from science to environmental management. Ecotoxicology and Environmental Safety, 147, pp. 430-446. [https://doi.org/10.1016/j.ecoenv.2017.08.064 DOI: 10.1016/j.ecoenv.2017.08.064]</ref>. These new methods, however, are likely to be considered supplemental to the more generalized framework shared globally.
| |
| − | | |
| − | ==Fundamentals of Sediment Risk Assessment==
| |
| − | [[File: SedRiskFig1.PNG | thumb |700px|Figure 1. Schematic of the sediment risk assessment process]]
| |
| − | Whereas there is strong evidence of anthropogenic impacts on the benthic community at many sediment sites, the degree of toxicity (or even its presence or absence) cannot be predicted with absolute certainty using contaminant concentrations alone<ref name="Apitz2011"/>. A sediment ERA should include lines of evidence (LOEs) derived from several different investigations<ref name="Wenning2005"/>. One common approach to develop several of these LOEs in a decision framework is the triad approach. Triad-based assessment frameworks require evidence based on sediment chemistry, toxicity, and benthic community structure (possibly including evidence of bioaccumulation) to designate sediment as toxic and requiring management or control<ref name="Chapman1996">Chapman, P.M., Paine, M.D., Arthur, A.D., Taylor, L.A., 1996. A triad study of sediment quality associated with a major, relatively untreated marine sewage discharge. Marine Pollution Bulletin 32(1), pp. 47–64. [https://doi.org/10.1016/0025-326X(95)00108-Y DOI: 10.1016/0025-326X(95)00108-Y]</ref>. In some decision frameworks, particularly those used to establish and rank risks in national or regional programs, all components of the triad are carried out simultaneously, with the various LOEs combined to support weight of evidence (WOE) decision making. In other frameworks, LOEs are tiered to minimize costs by collecting only the data required to make a decision and leaving some potential consequences and uncertainties unresolved.
| |
| − | | |
| − | Figure 1 provides an overview of a sediment risk assessment process. The first step, and a fundamental requirement, in sediment risk assessment, involves scoping and planning prior to undertaking work. This is important for optimizing the available assessment resource and conducting an assessment at the appropriate level of detail that is transparent and free, to the extent possible, of any bias or preconceived beliefs concerning the outcome<ref name="Hill2000">Hill, R.A., Chapman, P.M., Mann, G.S. and Lawrence, G.S., 2000. Level of Detail in Ecological Risk Assessments. Marine Pollution Bulletin, 40(6), pp. 471-477. [https://doi.org/10.1016/S0025-326X(00)00036-9 DOI: 10.1016/S0025-326X(00)00036-9]</ref>.
| |
| − | | |
| − | ===Screening-Level Risk Assessment (SLRA)===
| |
| − | Technical guidance in many countries strongly encourages sediment risk assessment to begin with a Screening-Level Risk Assessment (SLRA)<ref name="USEPA2005"/><ref name="Tarazona2014"/><ref name="Fletcher2008"/>. The SLRA is a simplified risk assessment conducted using limited data and often assuming certain, generally conservative and generic, sediment characteristics, sediment contaminant levels, and exposure parameters in the absence of sufficient readily available information<ref name="Hope2006">Hope, B.K., 2006. An examination of ecological risk assessment and management practices. Environment International, 32(8), pp. 983-995. [https://doi.org/10.1016/j.envint.2006.06.005 DOI: 10.1016/j.envint.2006.06.005]</ref><ref name="Weinstein2010">Weinstein, J.E., Crawford, K.D., Garner, T.R. and Flemming, A.J., 2010. Screening-level ecological and human health risk assessment of polycyclic aromatic hydrocarbons in stormwater detention pond sediments of Coastal South Carolina, USA. Journal of Hazardous Materials, 178(1-3), pp. 906-916. [https://doi.org/10.1016/j.jhazmat.2010.02.024 DOI: 10.1016/j.jhazmat.2010.02.024]</ref><ref name="Rak2008">Rak, A., Maly, M.E., Tracey, G., 2008. A Guide to Screening Level Ecological Risk Assessment, TG-090801. Tri-Services Ecological Risk Assessment Working Group (TSERAWG), U.S. Army Biological Technical Assistance Group (BTAG), Aberdeen Proving Ground, MD. 26 pp. [https://usaphcapps.amedd.army.mil/erawg/SLERA.pdf Free Download] [[Media: Rak2008.pdf | Report.pdf]]</ref><ref name="USEPA2001">US Environmental Protection Agency (USEPA), 2001. ECO Update. The Role of Screening-Level Risk Assessments and Refining Contaminants of Concern in Baseline Ecological Risk Assessments. EPA 540/F-01/014. Washington, D.C. [https://www.epa.gov/sites/default/files/2015-09/documents/slera0601.pdf Website] [[Media: EPA 540_F-01_014.pdf | Report.pdf]]</ref>.
| |
| − | | |
| − | The analysis is often semi-quantitative, and typically includes comparisons of various chemical and physical sediment conditions to threshold limits established in national or international regulations or by generally accepted scientific interpretations. US technical guidance encourages the comparison of contaminant measurements in water, sediment, or soil to National Oceanographic and Atmospheric Administration (NOAA) sediment screening quick reference tables, or SQuiRT cards, which list quality guidelines from a range of sources, based on differing narrative intent<ref name="Buchman2008">Buchman, M.F., 2008. Screening Quick Reference Tables (SQuiRTs), NOAA OR&R Report 08-1. National Oceanographic and Atmospheric Administration (NOAA), Coastal Protection and Restoration Protection Division. 34 pp. [https://repository.library.noaa.gov/view/noaa/9327 website] [[Media: SQuiRTs2008.pdf | Report.pdf]]</ref>.
| |
| − | | |
| − | The screening level approach is intended to minimize the chances of concluding that there is no risk when, in fact, risk may exist. Thus, the results of an SLRA may indicate contaminants or sediments in certain locations in the original study area initially thought to be of concern are acceptable (i.e., contaminant levels are below threshold levels), or that contaminant levels are high enough to indicate immediate action without further assessment (e.g., contaminant levels are well above probable-effects guidelines). In other cases, or at other locations, SLRA may indicate the need for further examination. Further study may apply site-specific, rather than generic and conservative assumptions, to reduce uncertainty.
| |
| − | | |
| − | ===Detailed Risk Assessment===
| |
| − | Detailed sediment risk assessment is conducted when SLRA results indicate one or more sediment contaminants exceed background conditions or regulatory threshold limits. For some contaminants, such as the dioxins and other persistent, bioaccumulative, and toxic substances (PBTs), technical guidance may mandate further examination, regardless of whether threshold levels are exceeded<ref name="Solomon2013">Solomon, K., Matthies, M., and Vighi, M., 2013. Assessment of PBTs in the European Union: a critical assessment of the proposed evaluation scheme with reference to plant protection products. Environmental Sciences Europe, 25(1), pp. 1-17. [https://doi.org/10.1186/2190-4715-25-10 DOI: 10.1186/2190-4715-25-10] [https://enveurope.springeropen.com/articles/10.1186/2190-4715-25-10 Open Access Article]</ref><ref name="Matthies2016">Matthies, M., Solomon, K., Vighi, M., Gilman, A. and Tarazona, J.V., 2016. The origin and evolution of assessment criteria for persistent, bioaccumulative and toxic (PBT) chemicals and persistent organic pollutants (POPs). Environmental Science: Processes and Impacts, 18(9), pp. 1114-1128. [https://doi.org/10.1039/C6EM00311G DOI: 10.1039/C6EM00311G]</ref>. Detailed sediment risk assessment typically follows a three-step framework similar to that described for ecological risk assessment - problem formulation, exposure analysis, and risk characterization<ref name="Suter2008">Suter, G.W., 2008. Ecological Risk Assessment in the United States Environmental Protection Agency: A Historical Overview. Integrated Environmental Assessment And Management, 4(3), pp. 285-289. [https://doi.org/10.1897/IEAM_2007-062.1 DOI: 10.1897/IEAM_2007-062.1] Free download from: [https://bioone.org/journals/integrated-environmental-assessment-and-management/volume-4/issue-3/IEAM_2007-062.1/Ecological-Risk-Assessment-in-the-United-States-Environmental-Protection-Agency/10.1897/IEAM_2007-062.1.pdf?casa_token=ieq3Cnc-YdIAAAAA:_MH-gpnwpJKvZSV2Qew43Y4ocdgADq1HvugpvmrblcGONMJgvIjYB52zQnXn_oAUW0gTy5GAkfY BioOne]</ref>.
| |
| − | | |
| − | US sediment management guidance describes a detailed risk assessment process similar to that followed for US ecological risk assessment<ref name="USEPA2005"/>. The first step is problem formulation. It involves defining chemical and physical conditions, delineating the spatial footprint of the sediment area to be examined, and identifying the human uses and ecological features of the sediment. Historical data are included in this initial step to better understand the results of biota, sediment, and water sampling as well as laboratory toxicity testing results. The SLRA is often included as a part of problem formulation.
| |
| − | | |
| − | The second step is exposure analysis. This step includes the identification of pathways by which human and aquatic organisms might directly or indirectly contact contaminants in the sediment. The exposure route (i.e., ingestion, dermal, or inhalation of particulates or gaseous emissions) and both the frequency and duration of contact (i.e., hourly, daily, or seasonally) are determined for each contaminant exposure pathway and human and ecological receptor. The environmental fate of the contaminant, factors affecting uptake, and the overall exposure dose are included in the calculation of the level of contaminant exposure. The exposure analysis also includes an effects assessment, whereby the biological response and associated level required to manifest different biological responses are determined for each contaminant.
| |
| − | | |
| − | The third step is risk-characterization. It involves calculating the risks for each human and ecological receptor posed by each sediment contaminant, as well as the cumulative risk associated with the combined exposure to all contaminants exerting similar biological effects. An uncertainty analysis is often included in this step of the risk assessment to convey where knowledge or data are lacking regarding the presence of the contaminant in the sediment, the biological response associated with exposure to the contaminant, or the behavior of the receptor with respect to contact with the sediment. A sensitivity analysis also may be conducted to convey the range of exposures (lowest, typical, and worst-case) and risks associated with changes in key assumptions and parameter values used in the exposure calculations and effects assessment.
| |
| − |
| |
| − | ==Key Considerations==
| |
| − | ===Stakeholder Engagement===
| |
| − | Stakeholder involvement is widely acknowledged as an important element of [[Wikipedia: Dredging | dredged]] material management<ref name="Collier2014">Collier, Z.A., Bates, M.E., Wood, M.D. and Linkov, I., 2014. Stakeholder engagement in dredged material management decisions. Science of the Total Environment, 496, pp. 248-256. [https://doi.org/10.1016/j.scitotenv.2014.07.044 DOI: 10.1016/j.scitotenv.2014.07.044] Free download from: [https://www.researchgate.net/profile/Matthew-Bates-9/publication/264460412_Stakeholder_Engagement_in_Dredged_Material_Management_Decisions/links/5a9d50fbaca2721e3f32adea/Stakeholder-Engagement-in-Dredged-Material-Management-Decisions.pdf ResearchGate]</ref>, sediment remediation<ref name="Oen2010">Oen, A.M.P., Sparrevik, M., Barton, D.N., Nagothu, U.S., Ellen, G.J., Breedveld, G.D., Skei, J. and Slob, A., 2010. Sediment and society: an approach for assessing management of contaminated sediments and stakeholder involvement in Norway. Journal of Soils and Sediments, 10(2), pp. 202-208. [https://doi.org/10.1007/s11368-009-0182-x DOI: 10.1007/s11368-009-0182-x]</ref>, and other environmental and sediment related activities<ref name="Gerrits2004">Gerrits, L. and Edelenbos, J., 2004. Management of Sediments Through Stakeholder Involvement. Journal of Soils and Sediments, 4(4), pp. 239-246. [https://doi.org/10.1007/BF02991120 DOI: 10.1007/BF02991120]</ref><ref name="Braun2019">Braun, A.B., da Silva Trentin, A.W., Visentin, C. and Thomé, A., 2019. Sustainable remediation through the risk management perspective and stakeholder involvement: A systematic and bibliometric view of the literature. Environmental Pollution, 255(1), p.113221. [https://doi.org/10.1016/j.envpol.2019.113221 DOI: 10.1016/j.envpol.2019.113221]</ref>.
| |
| − | | |
| − | Sediment management, particularly at the river basin scale, involves a wide variety of different environmental, governmental, and societal issues<ref name="Liu2018">Liu, C., Walling, D.E. and He, Y., 2018. The International Sediment Initiative case studies of sediment problems in river basins and their management. International Journal of Sediment Research, 33(2), pp. 216-219. [https://doi.org/10.1016/j.ijsrc.2017.05.005 DOI: 10.1016/j.ijsrc.2017.05.005] Free download from: [https://www.researchgate.net/profile/Cheng-Liu-43/publication/317032034_Review_The_International_Sediment_Initiative_Case_Studies_of_sediment_problems_in_river_basins_and_their_management/links/5f4f37d2299bf13a319703df/Review-The-International-Sediment-Initiative-Case-Studies-of-sediment-problems-in-river-basins-and-their-management.pdf ResearchGate]</ref>. Incorporating these different views, interests, and perspectives into a form that builds consensus for whatever actions and goals are in mind (e.g., commercial ports and shipping, navigation, flood protection, or habitat restoration) necessitates a formal stakeholder engagement process<ref name="Slob2008">Slob, A.F.L., Ellen, G.J. and Gerrits, L., 2008. Sediment management and stakeholder involvement. In: Sustainable Management of Sediment Resources, Vol. 4: Sediment Management at the River Basin Scale, Owens, P.N. (ed.), pp. 199-216. Elsevier. [https://doi.org/10.1016/S1872-1990(08)80009-8 DOI: 10.1016/S1872-1990(08)80009-8]</ref>.
| |
| − | | |
| − | Results from a three-year (2008-2010) [https://www.ngi.no/eng/Projects/Sediment-and-society Sediment and Society] research project funded by the Norwegian Research Council point to three important challenges that must be resolved for successful stakeholder engagement: (1) how to include people who have important management information and local knowledge, but not much influence in the decision-making process; (2) how to secure resources to ensure participation and (3) how to engage and motivate stakeholders to participate early in the sediment remediation planning process<ref name="Oen2010"/>.
| |
| − | | |
| − | ===Conceptual Site Model===
| |
| − | The preparation of a conceptual site model (CSM) is a fundamental component of problem formulation and the first step in detailed sediment risk assessment. The CSM is a narrative and/or illustrative representation of the physical, chemical and biological processes that control the transport, migration and actual or potential impacts of sediment contamination to human and/or ecological receptors<ref name="NJDEP2019">New Jersey Department of Environmental Protection, 2019. Technical Guidance for Preparation and Submission of a Conceptual Site Model. Version 1.1. Site Remediation and Waste Management Program, Trenton, NJ. 46 pp. [https://www.nj.gov/dep/srp/guidance/srra/csm_tech_guidance.pdf Free download].</ref><ref name="USEPA2011">US Environmental Protection Agency, 2011. Guidance for the Development of Conceptual Models for a Problem Formulation Developed for Registration Review. Environmental Fate and Effects Division, Office of Pesticide Programs, Washington, D.C. [https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/guidance-development-conceptual-models-problem Website]</ref>. The CSM should include a “food web” because the aquatic food web is an important exposure pathway by which contaminants in the sediment reach humans and pelagic aquatic life<ref name="Arnot2004">Arnot, J.A. and Gobas, F.A., 2004. A Food Web Bioaccumulation Model for Organic Chemicals in Aquatic Ecosystems. Environmental Toxicology and Chemistry, 23(10), pp. 2343-2355. [https://doi.org/10.1897/03-438 DOI: 10.1897/03-438]</ref>.
| |
| | | | |
| − | The CSM provides an early opportunity for critical examination of the interactions between sediment and the water column and the influence of groundwater inputs, surface runoff, and hydrodynamics. For example, there are situations where impacts in the aquatic food web can be driven by ongoing inputs to the water column from upstream sources, but mistakenly connected to polluted sediments. Other considerations included in a CSM can be socio-economic and include linkages to the ecosystem services provided by sediments<ref name="Broszeit2019">Broszeit, S., Beaumont, N.J., Hooper, T.L., Somerfield, P.J. and Austen, M.C., 2019. Developing conceptual models that link multiple ecosystem services to ecological research to aid management and policy, the UK marine example. Marine Pollution Bulletin, 141, pp.236-243. [https://doi.org/10.1016/j.marpolbul.2019.02.051 DOI: 10.1016/j.marpolbul.2019.02.051] [https://www.sciencedirect.com/science/article/pii/S0025326X19301511/pdfft?md5=34993d6c3a57b6fb18a8b6329597fcb9&pid=1-s2.0-S0025326X19301511-main.pdf Open Access Article.]</ref><ref name="Wang2021">Wang, J., Lautz, L.S., Nolte, T.M., Posthuma, L., Koopman, K.R., Leuven, R.S. and Hendriks, A.J., 2021. Towards a systematic method for assessing the impact of chemical pollution on ecosystem services of water systems. Journal of Environmental Management, 281, p. 111873. [https://doi.org/10.1016/j.jenvman.2020.111873 DOI: 10.1016/j.jenvman.2020.111873] [https://www.sciencedirect.com/science/article/pii/S0301479720317989/pdfft?md5=daff5e94f8aed44ffce6508afef2308c&pid=1-s2.0-S0301479720317989-main.pdf Open Access Article.]</ref>, or the social, economic and environmental impacts of sediment management alternatives. In such a case, when risk assessment seeks to compare risks of various management actions (including no action), the CSM can be termed a sustainability CSM, or SustCSM<ref name="McNally2020">McNally, A.D., Fitzpatrick, A.G., Harrison, D., Busey, A., and Apitz, S.E., 2020. Tiered approach to sustainability analysis in sediment remediation decision making. Remediation Journal, 31(1), pp. 29-44. [https://doi.org/10.1002/rem.21661 DOI: 10.1002/rem.21661] [https://onlinelibrary.wiley.com/doi/epdf/10.1002/rem.21661 Open Access Article].</ref><ref name="Holland2011">Holland, K.S., Lewis, R.E., Tipton, K., Karnis, S., Dona, C., Petrovskis, E., and Hook, C., 2011. Framework for Integrating Sustainability Into Remediation Projects. Remediation Journal, 21(3), pp. 7-38. [https://doi.org/10.1002/rem.20288 DOI: 10.1002/rem.20288].</ref>. At a minimum, however, the purpose of the CSM is to illustrate the scope of the risk assessment and guide the quantification of exposure and risk.
| + | ==Advantages and Disadvantages== |
| | | | |
| − | ===Environmental Fate=== | + | ===Advantages=== |
| − | An important consideration in exposure analysis is the determination of the bioavailable fraction of the contaminant in the sediment. There are two considerations. First, the adverse condition may be buried deep enough in sediments to be below the biologically available zone; typically, conditions in sediment below a depth of 5 cm will not contact burrowing benthic organisms<ref name="Anderson2010">Anderson, R.H., Prues, A.G. and Kravitz, M.J., 2010. Determination of the biologically relevant sampling depth for terrestrial ecological risk assessments. Geoderma, 154(3-4), pp.336-339. [https://doi.org/10.1016/j.geoderma.2009.11.004 DOI: 10.1016/j.geoderma.2009.11.004]</ref>. If there is no prospect for the adverse condition to come closer to the surface, then the risk assessment could conclude the risk of exposure is insignificant. The second consideration relates to chemistry and the factors involved in the binding to sediment particles or the chemical form of the substance in the sediment<ref name="Eggleton2004">Eggleton, J. and Thomas, K.V., 2004. A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environment International, 30(7), pp. 973-980. [https://doi.org/10.1016/j.envint.2004.03.001 DOI: 10.1016/j.envint.2004.03.001]</ref>. However, these assumptions should be examined in the context of [[Climate Change Primer | climate change]], and the likelihood of more frequent and extreme events, putting burial at risk, higher temperatures and changing biogeochemical conditions, which may alter environmental fate of contaminants, compared to historical studies.
| + | In comparison to other reported PFAS destruction techniques, PRD offers several advantages: |
| | + | *Relative to UV/sodium sulfite and UV/sodium iodide systems, the fitted degradation rates in the micelle-accelerated PRD reaction system were ~18 and ~36 times higher, indicating the key role of the self-assembled micelle in creating a confined space for rapid PFAS destruction<ref name="ChenEtAl2020"/>. The negatively charged hydrated electron associated with the positively charged cetyltrimethylammonium ion (CTA<sup>+</sup>) forms the surfactant micelle to trap molecules with similar structures, selectively mineralizing compounds with both hydrophobic and hydrophilic groups (e.g., PFAS). |
| | + | *The PRD reaction does not require solid catalysts or electrodes, which can be expensive to acquire and difficult to regenerate or dispose. |
| | + | *The aqueous solution is not heated or pressurized, and the UV wavelength used does not cause direct water [[Wikipedia: Photodissociation | photolysis]], therefore the energy input to the system is more directly employed to destroy PFAS, resulting in greater energy efficiency. |
| | + | *Since the reaction is performed at ambient temperature and pressure, there are limited concerns regarding environmental health and safety or volatilization of PFAS compared to heated and pressurized systems. |
| | + | *Due to the reductive nature of the reaction, there is no formation of unwanted byproducts resulting from oxidative processes, such as [[Wikipedia: Perchlorate | perchlorate]] generation during electrochemical oxidation<ref>Veciana, M., Bräunig, J., Farhat, A., Pype, M. L., Freguia, S., Carvalho, G., Keller, J., Ledezma, P., 2022. Electrochemical Oxidation Processes for PFAS Removal from Contaminated Water and Wastewater: Fundamentals, Gaps and Opportunities towards Practical Implementation. Journal of Hazardous Materials, 434, Article 128886. [https://doi.org/10.1016/j.jhazmat.2022.128886 doi: 10.1016/j.jhazmat.2022.128886]</ref><ref>Trojanowicz, M., Bojanowska-Czajka, A., Bartosiewicz, I., Kulisa, K., 2018. Advanced Oxidation/Reduction Processes Treatment for Aqueous Perfluorooctanoate (PFOA) and Perfluorooctanesulfonate (PFOS) – A Review of Recent Advances. Chemical Engineering Journal, 336, pp. 170–199. [https://doi.org/10.1016/j.cej.2017.10.153 doi: 10.1016/j.cej.2017.10.153]</ref><ref>Wanninayake, D.M., 2021. Comparison of Currently Available PFAS Remediation Technologies in Water: A Review. Journal of Environmental Management, 283, Article 111977. [https://doi.org/10.1016/j.jenvman.2021.111977 doi: 10.1016/j.jenvman.2021.111977]</ref>. |
| | + | *Aqueous fluoride ions are the primary end products of PRD, enabling real-time reaction monitoring with a fluoride [[Wikipedia: Ion-selective electrode | ion selective electrode (ISE)]], which is far less expensive and faster than relying on PFAS analytical data alone to monitor system performance. |
| | | | |
| − | The above contaminant bioavailability considerations are important factors influencing assumptions in the risk assessment about contaminant exposure<ref name="Peijnenburg2020">Peijnenburg, W.J., 2020. Implementation of bioavailability in prospective and retrospective risk assessment of chemicals in soils and sediments. In: The Handbook of Environmental Chemistry, vol 100, Bioavailability of Organic Chemicals in Soil and Sediment, Ortega-Calvo, J.J., Parsons, J.R. (ed.s), pp.391-422. Springer. [https://doi.org/10.1007/698_2020_516 DOI: 10.1007/698_2020_516]</ref><ref name="Ortega-Calvo2015">Ortega-Calvo, J.J., Harmsen, J., Parsons, J.R., Semple, K.T., Aitken, M.D., Ajao, C., Eadsforth, C., Galay-Burgos, M., Naidu, R., Oliver, R. and Peijnenburg, W.J., 2015. From Bioavailability Science to Regulation of Organic Chemicals. Environmental Science and Technology, 49, 10255−10264. [https://doi.org/10.1021/acs.est.5b02412 DOI: 10.1021/acs.est.5b02412] [https://pubs.acs.org/doi/pdf/10.1021/acs.est.5b02412 Open Access Article].</ref>. There have been recent advances in the use of sorbent amendments applied to contaminated sediments that alter sediment geochemistry, increase contaminant binding, and reduce contaminant exposure risks to people and the environment<ref name="Ghosh2011">Ghosh, U., Luthy, R.G., Cornelissen, G., Werner, D. and Menzie, C.A., 2011. In-situ sorbent amendments: a new direction in contaminated sediment management. Environmental Science and Technology, 45, 4, 1163–1168. [https://doi.org/10.1021/es102694h DOI: 10.1021/es102694h] [https://pubs.acs.org/doi/pdf/10.1021/es102694h Open Access Article]</ref>. [[Passive Sampling of Sediments | Passive sampling techniques]] have emerged to quantify chemical binding to sediment and determine the freely dissolved concentration that is bioavailable. | + | ===Disadvantages=== |
| | + | *The CTAB additive is only partially consumed during the reaction, and although CTAB is not problematic when discharged to downstream treatment processes that incorporate aerobic digestors, CTAB can be toxic to surface waters and anaerobic digestors. Therefore, disposal options for treated solutions will need to be evaluated on a site-specific basis. Possible options include removal of CTAB from solution for reuse in subsequent PRD treatments, or implementation of an oxidation reaction to degrade CTAB. |
| | + | *The PRD reaction rate decreases in water matrices with high levels of total dissolved solids (TDS). It is hypothesized that in high TDS solutions (e.g., ion exchange still bottoms with TDS of 200,000 ppm), the presence of ionic species inhibits the association of the electron donor with the micelle, thus decreasing the reaction rate. |
| | + | *The PRD reaction rate decreases in water matrices with very low UV transmissivity. Low UV transmissivity (i.e., < 1 %) prevents the penetration of UV light into the solution, such that the utilization efficiency of UV light decreases. |
| | | | |
| − | ===Assessment and Measurement Endpoints=== | + | ==State of the Art== |
| − | Assessment and measurement endpoints used in sediment risk assessment are comparable to those described in USEPA ecological risk assessment guidance<ref name="USEPA2005"/><ref name="USEPA1992">US Environmental Protection Agency (USEPA), 1992. Framework for Ecological Risk Assessment, EPA/630/R-92/001. Risk Assessment Forum, Washington DC. [[Media: EPA-630-R-92-001.pdf | Report.pdf]]</ref><ref name="USEPA1996">US Environmental Protection Agency (USEPA), 1996. Eco Update: Ecological Significance and Selection of Candidate Assessment Endpoints. EPA/540/F-95/037. Office of Solid Waste and Emergency Response, Washington DC. [[Media: EPA 540-F-95-037.pdf | Report.pdf]]</ref><ref name="USEPA1997b">US Environmental Protection Agency (USEPA), 1997. Ecological Risk Assessment Guidance for Superfund: Process for Designing and Conducting Ecological Risk Assessments - Interim Final, EPA 540/R-97/006. Office of Solid Waste and Emergency Response, Washington DC. [[Media: EPA 540-R-97-006.pdf | Report.pdf]]</ref><ref name="USEPA1998">US Environmental Protection Agency (USEPA), 1998. Guidelines for Ecological Risk Assessment. EPA/630/R-95/002F. Risk Assessment Forum, Washington DC. [[Media: EPA 630-R-95-002F.pdf | Report.pdf]]</ref>. A sediment risk assessment, and ecological risk assessments more broadly, must have clearly defined endpoints that are socially and biologically relevant, accessible to prediction and measurement, and susceptible to the hazard being assessed<ref name="USEPA1992"/>.
| |
| | | | |
| − | Assessment endpoints for humans include both carcinogenic and noncarcinogenic effects. Due to their assumed higher levels of exposure, human receptors used in sediment risk assessment typically include recreational, commercial, and subsistence fishermen, i.e., people who might be at increased risk from eating fish or contacting the sediment or water on a regular basis such as indigenous peoples, immigrants from fishing cultures, and subsistence fishers who rely upon fish as a major source of protein. Special considerations are given to women of child-bearing age, pregnant women and young children. Assessment endpoints for ecological receptors focus on benthic organisms, resident fish, piscivorous and other predatory birds and marine mammals. Endpoints typically include mortality, reproductive success and population susceptibility to disease or similar adverse chronic conditions.
| + | ===Technical Performance=== |
| | + | [[File:WittFig2.png | thumb |400px| Figure 2. Enspired Solutions<small><sup>TM</sup></small> commercial PRD PFAS destruction equipment, the PFASigator<small><sup>TM</sup></small>. Dimensions are 8 feet long by 4 feet wide by 9 feet tall.]] |
| | | | |
| − | Measurement endpoints are related quantitatively to each assessment endpoint. Whenever practical, multiple measurement endpoints are chosen to provide additional lines of evidence for each assessment endpoint. For example, for humans, it might be possible to measure contaminant levels in both food items and human blood or tissue. For predatory fish, birds and mammals, it might be possible to measure contaminants in both prey and predator tissues. Measurement endpoints can be selected to assess non-chemical stressors as well, such as habitat alteration and water turbidity. Typically, measurement endpoints are compared to measurements at a reference site to ascertain the degree of departure from local natural or background conditions.
| + | {| class="wikitable mw-collapsible" style="float:left; margin-right:20px; text-align:center;" |
| | + | |+Table 1. Percent decreases from initial PFAS concentrations during benchtop testing of PRD treatment in different water matrices |
| | + | |- |
| | + | ! Analytes |
| | + | ! |
| | + | ! GW |
| | + | ! FF |
| | + | ! AFFF<br>Rinsate |
| | + | ! AFF<br>(diluted 10X) |
| | + | ! IDW NF |
| | + | |- |
| | + | | Σ Total PFAS<small><sup>a</sup></small> (ND=0) |
| | + | | rowspan="9" style="background-color:white;" | <p style="writing-mode: vertical-rl">% Decrease<br>(Initial Concentration, μg/L)</p> |
| | + | | 93%<br>(370) || 96%<br>(32,000) || 89%<br>(57,000) || 86 %<br>(770,000) || 84%<br>(82) |
| | + | |- |
| | + | | Σ Total PFAS (ND=MDL) || 93%<br>(400) || 86%<br>(32,000) || 90%<br>(59,000) || 71%<br>(770,000) || 88%<br>(110) |
| | + | |- |
| | + | | Σ Total PFAS (ND=RL) || 94%<br>(460) || 96%<br>(32,000) || 91%<br>(66,000) || 34%<br>(770,000) || 92%<br>(170) |
| | + | |- |
| | + | | Σ Highly Regulated PFAS<small><sup>b</sup></small> (ND=0) || >99%<br>(180) || >99%<br>(20,000) || 95%<br>(20,000) || 92%<br>(390,000) || 95%<br>(50) |
| | + | |- |
| | + | | Σ Highly Regulated PFAS (ND=MDL) || >99%<br>(180) || 98%<br>(20,000) || 95%<br>(20,000) || 88%<br>(390,000) || 95%<br> (52) |
| | + | |- |
| | + | | Σ Highly Regulated PFAS (ND=RL) || >99%<br>(190) || 93%<br>(20,000) || 95%<br>(20,000) || 79%<br>(390,000) || 95%<br>(55) |
| | + | |- |
| | + | | Σ High Priority PFAS<small><sup>c</sup></small> (ND=0) || 91%<br>(180) || 98%<br>(20,000) || 85%<br>(20,000) || 82%<br>(400,000) || 94%<br>(53) |
| | + | |- |
| | + | | Σ High Priority PFAS (ND=MDL) || 91%<br>(190) || 94%<br>(20,000) || 85%<br>(20,000) || 79%<br>(400,000) || 86%<br>(58) |
| | + | |- |
| | + | | Σ High Priority PFAS (ND=RL) || 92%<br>(200) || 87%<br>(20,000) || 86%<br>(21,000) || 70%<br>(400,000) || 87%<br>(65) |
| | + | |- |
| | + | | Fluorine mass balance<small><sup>d</sup></small> || ||106% || 109% || 110% || 65% || 98% |
| | + | |- |
| | + | | Sorbed organic fluorine<small><sup>e</sup></small> || || 4% || 4% || 33% || N/A || 31% |
| | + | |- |
| | + | | colspan="7" style="background-color:white; text-align:left" | <small>Notes:<br>GW = groundwater<br>GW FF = groundwater foam fractionate<br>AFFF rinsate = rinsate collected from fire system decontamination<br>AFFF (diluted 10x) = 3M Lightwater AFFF diluted 10x<br>IDW NF = investigation derived waste nanofiltrate<br>ND = non-detect<br>MDL = Method Detection Limit<br>RL = Reporting Limit<br><small><sup>a</sup></small>Total PFAS = 40 analytes + unidentified PFCA precursors<br><small><sup>b</sup></small>Highly regulated PFAS = PFNA, PFOA, PFOS, PFHxS, PFBS, HFPO-DA<br><small><sup>c</sup></small>High priority PFAS = PFNA, PFOA, PFHxA, PFBA, PFOS, PFHxS, PFBS, HFPO-DA<br><small><sup>d</sup></small>Ratio of the final to the initial organic fluorine plus inorganic fluoride concentrations<br><small><sup>e</sup></small>Percent of organic fluorine that sorbed to the reactor walls during treatment<br></small> |
| | + | |} |
| | + | </br> |
| | + | The PRD reaction has been validated at the bench scale for the destruction of PFAS in a variety of environmental samples from Department of Defense sites (Table 1). Enspired Solutions<small><sup>TM</sup></small> has designed and manufactured a fully automatic commercial-scale piece of equipment called PFASigator<small><sup>TM</sup></small>, specializing in PRD PFAS destruction (Figure 2). This equipment is modular and scalable, has a small footprint, and can be used alone or in series with existing water treatment trains. The PFASigator<small><sup>TM</sup></small> employs commercially available UV reactors and monitoring meters that have been used in the water industry for decades. The system has been tested on PRD efficiency operational parameters, and key metrics were proven to be consistent with benchtop studies. |
| | | | |
| − | ===Sediment Toxicity Testing===
| + | Bench scale PRD tests were performed for the following samples collected from Department of Defense sites: groundwater (GW), groundwater foam fractionate (FF), firefighting truck rinsate ([[Wikipedia: Firefighting foam | AFFF]] Rinsate), 3M Lightwater AFFF, investigation derived waste nanofiltrate (IDW NF), [[Wikipedia: Ion exchange | ion exchange]] still bottom (IX SB), and Ansulite AFFF. The PRD treatment was more effective in low conductivity/TDS solutions. Generally, PRD reaction rates decrease for solutions with a TDS > 10,000 ppm, with an upper limit of 30,000 ppm. Ansulite AFFF and IX SB samples showed low destruction efficiencies during initial screening tests, which was primarily attributed to their high TDS concentrations. Benchtop testing data are shown in Table 1 for the remaining five sample matrices. |
| − | Sediment bioassays are an integral part of effects characterization when assessing the risks posed by contaminated sediments and developing sediment quality guidelines<ref name="USEPA2014">US Environmental Protection Agency (USEPA), 2014. Toxicity Testing and Ecological Risk Assessment Guidance for Benthic Invertebrates. Office of Chemical Safety and Pollution Prevention, Washington DC. [https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/toxicity-testing-and-ecological-risk-assessment Website] [[Media: USEPA2014.pdf | Report.pdf]]</ref><ref name="Simpson2016a">Simpson, S., Campana, O., Ho, K., 2016. Chapter 7, Sediment Toxicity Testing. In: J. Blasco, P.M. Chapman, O. Campana, M. Hampel (ed.s), Marine Ecotoxicology: Current Knowledge and Future Issues. Academic Press Incorporated. pp. 199-237. [https://doi.org/10.1016/B978-0-12-803371-5.00007-2 DOI: 10.1016/B978-0-12-803371-5.00007-2]</ref>. The selection of appropriate sediment bioassays is dependent on the questions being addressed, the physical and chemical characteristics of the sediment matrix, the nature of the contaminant(s) of concern, and preferences of the supervising regulatory authority for the test method and test organisms<ref name="Amiard-Triquet2015">Amiard-Triquet, C., Amiard, J.C. and Mouneyrac, C. (ed.s), 2015. Aquatic Ecotoxicology: Advancing Tools For Dealing With Emerging Risks. Academic Press, NY. ISBN #9780128009499. [https://doi.org/10.1016/B978-0-12-800949-9.12001-7 DOI: 10.1016/B978-0-12-800949-9.12001-7]</ref>. Bioassay procedures have been standardized in several countries, and it is not unusual for different test methods to be required in different countries for the same sediment management purpose<ref name="DelValls2004">DelValls, T.A., Andres, A., Belzunce, M.J., Buceta, J.L., Casado-Martinez, M.C., Castro, R., Riba, I., Viguri, J.R. and Blasco, J., 2004. Chemical and ecotoxicological guidelines for managing disposal of dredged material. TrAC Trends in Analytical Chemistry, 23(10-11), pp. 819-828. [https://doi.org/10.1016/j.trac.2004.07.014 DOI: 10.1016/j.trac.2004.07.014] Free download from: [https://d1wqtxts1xzle7.cloudfront.net/46085251/Chemical_and_Ecotoxicological_Guidelines20160530-23122-4fooj2-with-cover-page-v2.pdf?Expires=1637618385&Signature=aNsOfciO0HPhucL8S713nenRlvviD2dbLi8y63n93iGX~Cc7CHwyYQ2bfNlT6VnjuFJeVT83M01Xog6esr14gyvL9pmlo3hw5fQp5J9vA8gqXcT9kQfM1T2Q0Ig883yGMFmtgUrrU6p8c8V~8rh5DTKDD5ZsiL4zloGgF6Gs4F2ecEDqyFBZ17yYpXGVVBmpfm87sUpaPY0Ix9iWJ~5nxM~HF6XYl1sA1rgFSerT-Y5W8Ma7-XMljnYHQ7hW7eqMjyN66IDj7pwafG7Ox-Hnp07IuD-oMY1dHHrzTOmHpXpWgMYLn2zf1BSmy~tqIFHE6UjZn5ako93PgExuzEjEiw__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA Academia.edu]</ref>. Guidance documents in Australia, Canada, Europe and the US cover the wide range of sediment bioassay procedures most often used in risk assessment<ref name="Bat2005">Bat, L., 2005. A Review of Sediment Toxicity Bioassays Using the Amphipods and Polychaetes. Turkish Journal of Fisheries and Aquatic Sciences, 5(2), pp. 119-139. [https://dergipark.org.tr/en/pub/trjfas-ayrildi/issue/13287/160604 Free download] [[Media: Bat2005.pdf | Report.pdf]]</ref><ref name="Keddy1995">Keddy, C.J., Greene, J.C. and Bonnell, M.A., 1995. Review of Whole-Organism Bioassays: Soil, Freshwater Sediment, and Freshwater Assessment in Canada. Ecotoxicology and Environmental Safety, 30(3), pp. 221-251. [https://doi.org/10.1006/eesa.1995.1027 DOI: 10.1006/eesa.1995.1027]</ref><ref name="Giesy1990">Giesy, J.P., Rosiu, C.J., Graney, R.L. and Henry, M.G., 1990. Benthic invertebrate bioassays with toxic sediment and pore water. Environmental Toxicology and Chemistry, 9(2), pp. 233-248. [https://doi.org/10.1002/etc.5620090214 DOI: 10.1002/etc.5620090214]</ref><ref name="Simpson2016b">Simpson, S. and Batley, G. (ed.s), 2016. Sediment Quality Assessment: A Practical Guide, Second Edition. 358 pp. CSIRO Publishing, Australia. ISBN # 9781486303847.</ref><ref name="Moore2019">Moore, D.W., Farrar, D., Altman, S. and Bridges, T.S., 2019. Comparison of Acute and Chronic Toxicity Laboratory Bioassay Endpoints with Benthic Community Responses in Field‐Exposed Contaminated Sediments. Environmental Toxicology and Chemistry, 38(8), pp. 1784-1802. [https://doi.org/10.1002/etc.4454 DOI: 10.1002/etc.4454]</ref>.
| |
| | | | |
| − | In general, sediment toxicity tests focus on either (acute) lethality in whole organisms (typically benthic infaunal species such as amphipods and polychaetes) following short-term or acute exposures (<14 days) or (chronic) sublethal responses (e.g., reduced growth or reproduction or both) following longer-term exposures<ref name="Simpson2016a"/>. It is not unusual in sediment risk assessment to rely on more than one sediment bioassay. Both acute and chronic tests involving either solid-phase or pore-water sediment fractions can be useful to discern the contributions of different contaminants in whole sediment by examining the response of different endpoints in different test organisms<ref name="Keddy1995"/><ref name="Giesy1990"/>. The application of more specialized techniques such as toxicity identification evaluations (TIEs) have also proved useful to help identify contaminants or contaminant classes most likely responsible for toxicity and to exclude potentially confounding factors such as ammonia<ref name="Ho2013">Ho, K.T. and Burgess, R.M., 2013. What's causing toxicity in sediments? Results of 20 years of toxicity identification and evaluations. Environmental Toxicology and Chemistry, 32(11), pp. 2424-2432. [https://doi.org/10.1002/etc.2359 DOI: 10.1002/etc.2359]</ref><ref name="Bailey2016">Bailey, H.C., Curran, C.A., Arth, P., Lo, B.P. and Gossett, R., 2016. Application of sediment toxicity identification evaluation techniques to a site with multiple contaminants. Environmental Toxicology and Chemistry, 35(10), pp. 2456-2465. [https://doi.org/10.1002/etc.3488 DOI: 10.1002/etc.3488]</ref>.
| + | During treatment, PFOS and PFOA concentrations decreased 96% to >99% and 77% to 97%, respectively. For the PFAS with proposed drinking water Maximum Contaminant Levels (MCLs) recently established by the USEPA (PFNA, PFOA, PFOS, PFHxS, PFBS, and HFPO-DA), concentrations decreased >99% for GW, 93% for FF, 95% for AFFF Rinsate and IDW NF, and 79% for AFFF (diluted 10x) during the treatment time allotted. Meanwhile, the total PFAS concentrations, including all 40 known PFAS analytes and unidentified perfluorocarboxylic acid (PFCA) precursors, decreased from 34% to 96% following treatment. All of these concentration reduction values were calculated by using reporting limits (RL) as the concentrations for non-detects. |
| | | | |
| − | ===Uncertainty===
| + | Excellent fluorine/fluoride mass balance was achieved. There was nearly a 1:1 conversion of organic fluorine to free inorganic fluoride ion during treatment of GW, FF and AFFF Rinsate. The 3M Lightwater AFFF (diluted 10x) achieved only 65% fluorine mass balance, but this was likely due to high adsorption of PFAS to the reactor. |
| − | As part of the overall analysis of risk from exposure to certain sediment conditions, it is generally understood there is a moderate degree of uncertainty associated with sampling and the environmental fate of contaminants; an order of magnitude of uncertainty associated with ecological exposure and dose-response; and greater than an order of magnitude of uncertainty associated with the quantification of potential human health effects
| |
| | | | |
| − | ==Cap Design and Materials for Habitat Restoration== | + | ===Application=== |
| − | In addition to providing chemical isolation and containment, a cap can also be used to provide improvements for organisms by enhancing the habitat characteristics of the bottom substrate<ref name="Yozzo2004">Yozzo, D.J., Wilber, P. and Will, R.J., 2004. Beneficial use of dredged material for habitat creation, enhancement, and restoration in New York–New Jersey Harbor. Journal of Environmental Management, 73(1), pp. 39-52. [https://doi.org/10.1016/j.jenvman.2004.05.008 DOI: 10.1016/j.jenvman.2004.05.008]</ref><ref name="Zhang2016">Zhang, C., Zhu, M.Y., Zeng, G.M., Yu, Z.G., Cui, F., Yang, Z.Z. and Shen, L.Q., 2016. Active capping technology: a new environmental remediation of contaminated sediment. Environmental Science and Pollution Research, 23(5), pp.4370-4386. [https://doi.org/10.1007/s11356-016-6076-8 DOI: 10.1007/s11356-016-6076-8]</ref><ref name="Vlassopoulos2017"/>. Often, contaminated sediment environments are degraded for a variety of reasons in addition to the toxic constituents. One way to overcome this is to provide both a habitat layer and chemical isolation or contaminant capping layer. Figure 2 illustrates just such a design providing a more appropriate habitat enhancing substrate, in this case by incorporation additional organic material, vegetation and debris, which is often used by fish species for protection, into the surface layer. In a high energy environment, it should be recognized that it may not be possible to keep a suitable habitat layer in place during high flow events. This would be true of suitable habitat that had developed naturally as well as a constructed habitat layer and it is presumed that if such a habitat is the normal condition of the waterbody that it will recover over time between such high flow events.
| + | Due to the first-order kinetics of PRD, destruction of PFAS is most energy efficient when paired with a pre-concentration technology, such as foam fractionation (FF), nanofiltration, reverse osmosis, or resin/carbon adsorption, that remove PFAS from water. Application of the PFASigator<small><sup>TM</sup></small> is therefore proposed as a part of a PFAS treatment train that includes a pre-concentration step. |
| | | | |
| − | ==Summary==
| + | The first pilot study with the PFASigator<small><sup>TM</sup></small> was conducted in late 2023 at an industrial facility in Michigan with PFAS-impacted groundwater. The goal of the pilot study was to treat the groundwater to below the limits for regulatory discharge permits. For the pilot demonstration, the PFASigator<small><sup>TM</sup></small> was paired with an FF unit, which pre-concentrated the PFAS into a foamate that was pumped into the PFASigator<small><sup>TM</sup></small> for batch PFAS destruction. Residual PFAS remaining after the destruction batch was treated by looping back the PFASigator<small><sup>TM</sup></small> effluent to the FF system influent. During the one-month field pilot duration, site-specific discharge limits were met, and steady state operation between the FF unit and PFASigator<small><sup>TM</sup></small> was achieved such that the PFASigator<small><sup>TM</sup></small> destroyed the required concentrated PFAS mass and no off-site disposal of PFAS contaminated waste was required. |
| − | Clean substrate can be placed at the sediment-water interface for the purposes of reducing exposure to and risk from contaminants in the sediments. The cap can consist of simple materials such as sand designed to physically stabilize contaminated sediments and separate the benthic community from those contaminants or may include other materials designed to sequester contaminants even under adverse conditions including strong groundwater upwelling or highly mobile contaminants. The surface of a cap may be designed of coarse material such as gravel or cobble to be stable under high flow events or designed to be more appropriate habitat for benthic and aquatic organisms. As a result of its flexibility, simplicity and low cost relative to its effectiveness, capping is one of the most prevalent remedial technologies for sediments.
| |
| | | | |
| | ==References== | | ==References== |