Munitions Constituents - Abiotic Reduction
Munition compounds (MCs) often contain one or more nitro (-NO2) functional groups which makes them susceptible to abiotic reduction, i.e., transformation by accepting electrons from a chemical electron donor. In soil and groundwater, the most prevalent electron donors are natural organic carbon and iron minerals. Understanding the kinetics and mechanisms of abiotic reduction of MCs by carbon and iron constituents in soil is not only essential for evaluating the environmental fate of MCs but also key to developing cost-efficient remediation strategies. This article summarizes the recent advances in our understanding of MC reduction by carbon and iron based reductants.
Related Article(s):
- Munitions Constituents
- Munitions Constituents - Alkaline Degradation
- Munitions Constituents - Composting
- Munitions Constituents - Deposition
- Munitions Constituents - Dissolution
- Munitions Constituents - Electrochemical Treatment
- Munitions Constituents - Photolysis
- Munitions Constituents - Sorption
Contributor(s): Dr. Jimmy Murillo-Gelvez, Paula Andrea Cárdenas-Hernández, Dr. Dominic M. Di Toro, Dr. Richard F. Carbonaro and Dr. Pei Chiu
Key Resource(s):
- Schwarzenbach, Gschwend, and Imboden, 2016. Environmental Organic Chemistry, 3rd ed.[1]
Introduction
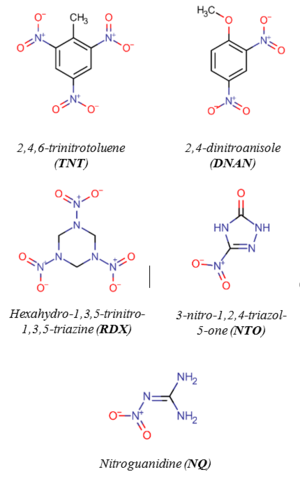
Legacy and insensitive MCs (Figure 1) are susceptible to reductive transformation in soil and groundwater. Many redox-active constituents in the subsurface, especially those containing organic carbon, Fe(II), and sulfur can mediate MC reduction. Specific examples include Fe(II)-organic complexes[2][3][4][5][6], iron oxides in the presence of aqueous Fe(II)[7][8][9][10][11][12][13][14][15][16][17], magnetite[12][14][18][19][20], Fe(II)-bearing clays[21][22][23][24][25][26][27], hydroquinones (as surrogates of natural organic matter)[4][28][29][30][31][32][33], dissolved organic matter[34][35][36], black carbon[37][38][39][40][41][42], and sulfides[43][44]. These geo-reductants may control the fate and half-lives of MCs in the environment and can be used to promote MC degradation in soil and groundwater through enhanced natural attenuation[45].
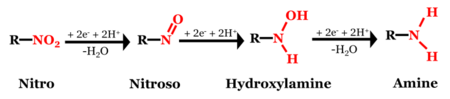
Although the chemical structures of MCs can vary significantly (Figure 1), most of them contain at least one nitro functional group (-NO2), which is susceptible to reductive transformation[46]. Of the MCs shown in Figure 1, 2,4,6-trinitrotoluene (TNT), 2,4-dinitroanisole (DNAN), and 3-nitro-1,2,4-triazol-5-one (NTO)[47] are nitroaromatic compounds (NACs) and hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and nitroguanidine (NQ) are nitramines. The structural differences may result in different reactivities and reaction pathways. Reduction of NACs results in the formation of aromatic amines (i.e., anilines) with nitroso and hydroxylamine compounds as intermediates (Figure 2)[1].
Although the final reduction products are different for non-aromatic MCs, the reduction process often starts with the transformation of the -NO2 moiety, either through de-nitration (e.g., RDX[48][49]) or reduction to nitroso[33][50] followed by ring cleavage[6][49][50][51].
Figure 3 illustrates a typical MC reduction reaction. A redox-active soil constituent, such as organic matter or iron mineral, donates electrons to an MC and transforms the nitro group into an amino group (R-NH2). The rate at which an MC is reduced can vary by many orders of magnitude depending on the soil constituent, the MC, the reduction potential (EH) and other media conditions[52].
The most prevalent reductants in soils are iron minerals and organic carbon such as that found in natural organic matter. It has been suggested that Fe(II)aq and dissolved organic matter concentrations could serve as indicators of NAC reducibility in anaerobic sediments[53]. The following sections summarize these two classes of reductants separately and present advances in our understanding of the kinetics of NAC/MC reduction by these geo-reductants.
Carbonaceous Reductants
The two most predominant forms of organic carbon in natural systems are natural organic matter (NOM) and black carbon (BC)[54]. Black carbon includes charcoal, soot, graphite, and coal. Until the early 2000s black carbon was considered to be a class of (bio)chemically inert geosorbents[55]. However, it has been shown that BC can contain abundant quinone functional groups and thus can store and exchange electrons[56] with chemical[57] and biological[58] agents in the surroundings. Specifically, BC such as biochar has been shown to reductively transform MCs including NTO, DNAN, and RDX[42].
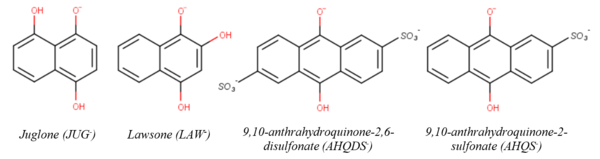
NOM encompasses all the organic compounds present in terrestrial and aquatic environments and can be classified into two groups, non-humic and humic substances. Humic substances (HS) contain a wide array of functional groups including carboxyl, enol, ether, ketone, ester, amide, (hydro)quinone, and phenol[59]. Quinone and hydroquinone groups are believed to be the predominant redox moieties responsible for the capacity of HS and BC to store and reversibly accept and donate electrons (i.e., through reduction and oxidation of HS/BC, respectively)[28][34][56][60][61][62][63][64][65][66][67][68][69][70][71].
Hydroquinones have been widely used as surrogates to understand the reductive transformation of NACs and MCs by NOM. Figure 4 shows the chemical structures of the singly deprotonated forms of four hydroquinone species previously used to study NAC/MC reduction. The second-order rate constants (kR) for the reduction of NACs/MCs by these hydroquinone species are listed in Table 1, along with the aqueous-phase one electron reduction potentials of the NACs/MCs (EH1’) where available. EH1’ is an experimentally measurable thermodynamic property that reflects the propensity of a given NAC/MC to accept an electron in water (EH1(R-NO2)):
- Equation 1: R-NO2 + e- ⇔ R-NO2•-
Knowing the identity of and reaction order in the reductant is required to derive the second-order rate constants listed in Table 1. This same reason limits the utility of reduction rate constants measured with complex carbonaceous reductants such as NOM[34], BC[37][38][39][72], and HS[35][36], whose chemical structures and redox moieties responsible for the reduction, as well as their abundance, are not clearly defined or known. In other words, the observed rate constants in those studies are specific to the experimental conditions (e.g., pH and NOM source and concentration), and may not be easily comparable to other studies.
| Compound | EH1' (V) | Hydroquinone [log kR (M-1s-1)] | |||
|---|---|---|---|---|---|
| (NAC/MC) | LAW- | JUG- | AHQDS- | AHQS- | |
| Nitrobenzene (NB) | -0.485[28] | 0.380[28] | -1.102[28] | 2.050[31] | 3.060[31] |
| 2-nitrotoluene (2-NT) | -0.590[28] | -1.432[28] | -2.523[28] | 0.775[4] | |
| 3-nitrotoluene (3-NT) | -0.475[28] | 0.462[28] | -0.921[28] | ||
| 4-nitrotoluene (4-NT) | -0.500[28] | 0.041[28] | -1.292[28] | 1.822[4] | 2.610[31] |
| 2-chloronitrobenzene (2-ClNB) | -0.485[28] | 0.342[28] | -0.824[28] | 2.412[4] | |
| 3-chloronitrobenzene (3-ClNB) | -0.405[28] | 1.491[28] | 0.114[28] | ||
| 4-chloronitrobenzene (4-ClNB) | -0.450[28] | 1.041[28] | -0.301[28] | 2.988[4] | |
| 2-acetylnitrobenzene (2-AcNB) | -0.470[28] | 0.519[28] | -0.456[28] | ||
| 3-acetylnitrobenzene (3-AcNB) | -0.405[28] | 1.663[28] | 0.398[28] | ||
| 4-acetylnitrobenzene (4-AcNB) | -0.360[28] | 2.519[28] | 1.477[28] | ||
| 2-nitrophenol (2-NP) | 0.568 (0.079)[28] | ||||
| 4-nitrophenol (4-NP) | -0.699 (-1.301)[28] | ||||
| 4-methyl-2-nitrophenol (4-Me-2-NP) | 0.748 (0.146)[28] | ||||
| 4-chloro-2-nitrophenol (4-Cl-2-NP) | 1.602 (1.114)[28] | ||||
| 5-fluoro-2-nitrophenol (5-Cl-2-NP) | 0.447 (-0.155)[28] | ||||
| 2,4,6-trinitrotoluene (TNT) | -0.280[1] | 2.869[30] | 5.204[4] | ||
| 2-amino-4,6-dinitrotoluene (2-A-4,6-DNT) | -0.400[1] | 0.987[30] | |||
| 4-amino-2,6-dinitrotoluene (4-A-2,6-DNT) | -0.440[1] | 0.079[30] | |||
| 2,4-diamino-6-nitrotoluene (2,4-DA-6-NT) | -0.505[1] | -1.678[30] | |||
| 2,6-diamino-4-nitrotoluene (2,6-DA-4-NT) | -0.495[1] | -1.252[30] | |||
| 1,3-dinitrobenzene (1,3-DNB) | -0.345[30] | 1.785[30] | |||
| 1,4-dinitrobenzene (1,4-DNB) | -0.257[30] | 3.839[30] | |||
| 2-nitroaniline (2-NANE) | < -0.560[30] | -2.638[30] | |||
| 3-nitroaniline (3-NANE) | -0.500[30] | -1.367[30] | |||
| 1,2-dinitrobenzene (1,2-DNB) | -0.290[30] | 5.407[4] | |||
| 4-nitroanisole (4-NAN) | -0.661[31] | 1.220[31] | |||
| 2-amino-4-nitroanisole (2-A-4-NAN) | -0.924[31] | 1.150[31] | 2.190[31] | ||
| 4-amino-2-nitroanisole (4-A-2-NAN) | 1.610[31] | 2.360[31] | |||
| 2-chloro-4-nitroaniline (2-Cl-5-NANE) | -0.863[31] | 1.250[31] | 2.210[31] | ||
| N-methyl-4-nitroaniline (MNA) | -1.740[31] | -0.260[31] | 0.692[31] | ||
| 3-nitro-1,2,4-triazol-5-one (NTO) | 5.701 (1.914)[36] | ||||
| Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) | -0.349[48] | ||||
Most of the current knowledge about MC degradation is derived from studies using NACs. The reduction kinetics of only four MCs, namely TNT, N-methyl-4-nitroaniline (MNA), NTO, and RDX, have been investigated with hydroquinones. Of these four MCs, only the reduction rates of MNA and TNT have been modeled[30][31][73][74].
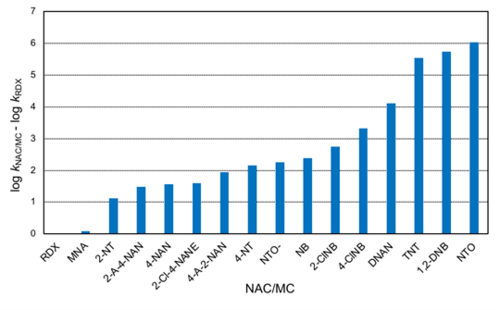
Using the rate constants obtained with AHQDS–, a relative reactivity trend can be obtained (Figure 5). RDX is the slowest reacting MC in Table 1, hence it was selected to calculate the relative rates of reaction (i.e., log kNAC/MC – log kRDX). If only the MCs in Figure 5 are considered, the reactivity spans 6 orders of magnitude following the trend: RDX ≈ MNA < NTO– < DNAN < TNT < NTO. The rate constant for DNAN reduction by AHQDS– is not yet published and hence not included in Table 1. Note that speciation of NACs/MCs can significantly affect their reduction rates. Upon deprotonation, the NAC/MC becomes negatively charged and less reactive as an oxidant (i.e., less prone to accept an electron). As a result, the second-order rate constant can decrease by 0.5-0.6 log unit in the case of nitrophenols and approximately 4 log units in the case of NTO (numbers in parentheses in Table 1)[28][36].
Ferruginous Reductants
| Compound | EH1' (V) | Cysteine[3] [FeL2]2- |
Thioglycolic acid[3] [FeL2]2- |
DFOB[5] [FeHL]0 |
AcHA[5] [FeL3]- |
Tiron a [FeL2]6- |
Fe-Porphyrin b |
|---|---|---|---|---|---|---|---|
| Fe(II)-Ligand [log kR (M-1s-1)] | |||||||
| Nitrobenzene | -0.485[28] | -0.347 | 0.874 | 2.235 | -0.136 | 1.424[75] 4.000[74] |
-0.018[28] 0.026[74] |
| 2-nitrotoluene | -0.590[28] | -0.602[28] | |||||
| 3-nitrotoluene | -0.475[28] | -0.434 | 0.767 | 2.106 | -0.229 | 1.999[75] 3.800[74] |
0.041[28] |
| 4-nitrotoluene | -0.500[28] | -0.652 | 0.528 | 2.013 | -0.402 | 1.446[75] 3.500[74] |
-0.174[28] |
| 2-chloronitrobenzene | -0.485[28] | 0.944[28] | |||||
| 3-chloronitrobenzene | -0.405[28] | 0.360 | 1.810 | 2.888 | 0.691 | 2.882[75] 4.900[74] |
0.724[28] |
| 4-chloronitrobenzene | -0.450[28] | 0.230 | 1.415 | 2.512 | 0.375 | 3.937[75] 4.581[2] |
0.431[28] 0.289[74] |
| 2-acetylnitrobenzene | -0.470[28] | 1.377[28] | |||||
| 3-acetylnitrobenzene | -0.405[28] | 0.799[28] | |||||
| 4-acetylnitrobenzene | -0.360[28] | 0.965 | 2.771 | 1.872 | 5.028[75] 6.300[74] |
1.286[28] | |
| RDX | -0.550[76] | 2.212[75] 2.864[6] |
|||||
| HMX | -0.660[76] | -2.762[75] | |||||
| TNT | -0.280[1] | 7.427[75] | 2.050[74] | ||||
| 1,3-dinitrobenzene | -0.345[30] | 1.220[74] | |||||
| 2,4-dinitrotoluene | -0.380[1] | 5.319[75] | 1.156[74] | ||||
| Nitroguanidine (NQ) | -0.700[76] | -0.185[75] | |||||
| 2,4-dinitroanisole (DNAN) | -0.400[76] | 1.243[74] | |||||
| Notes: a 4,5-dihydroxybenzene-1,3-disulfonate (Tiron). b meso-tetra(N-methyl-pyridyl)iron porphin in cysteine. | |||||||
| MC | Iron Mineral | Iron mineral loading (g/L) |
Surface area (m2/g) |
Fe(II)aq initial (mM) b |
Fe(II)aq after 24 h (mM) c |
Fe(II)aq sorbed (mM) d |
pH | Buffer | Buffer (mM) |
MC initial (μM) e |
log kobs (h-1) f |
log kSA (Lh-1m-2) g |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TNT[30] | Goethite | 0.64 | 17.5 | 1.5 | 7.0 | MOPS | 25 | 50 | 1.200 | 0.170 | ||
| RDX[77] | Magnetite | 1.00 | 44 | 0.1 | 0 | 0.10 | 7.0 | HEPES | 50 | 50 | -3.500 | -5.200 |
| RDX[77] | Magnetite | 1.00 | 44 | 0.2 | 0.02 | 0.18 | 7.0 | HEPES | 50 | 50 | -2.900 | -4.500 |
| RDX[77] | Magnetite | 1.00 | 44 | 0.5 | 0.23 | 0.27 | 7.0 | HEPES | 50 | 50 | -1.900 | -3.600 |
| RDX[77] | Magnetite | 1.00 | 44 | 1.5 | 0.94 | 0.56 | 7.0 | HEPES | 50 | 50 | -1.400 | -3.100 |
| RDX[77] | Magnetite | 1.00 | 44 | 3.0 | 1.74 | 1.26 | 7.0 | HEPES | 50 | 50 | -1.200 | -2.900 |
| RDX[77] | Magnetite | 1.00 | 44 | 5.0 | 3.38 | 1.62 | 7.0 | HEPES | 50 | 50 | -1.100 | -2.800 |
| RDX[77] | Magnetite | 1.00 | 44 | 10.0 | 7.77 | 2.23 | 7.0 | HEPES | 50 | 50 | -1.000 | -2.600 |
| RDX[77] | Magnetite | 1.00 | 44 | 1.6 | 1.42 | 0.16 | 6.0 | MES | 50 | 50 | -2.700 | -4.300 |
| RDX[77] | Magnetite | 1.00 | 44 | 1.6 | 1.34 | 0.24 | 6.5 | MOPS | 50 | 50 | -1.800 | -3.400 |
| RDX[77] | Magnetite | 1.00 | 44 | 1.6 | 1.21 | 0.37 | 7.0 | MOPS | 50 | 50 | -1.200 | -2.900 |
| RDX[77] | Magnetite | 1.00 | 44 | 1.6 | 1.01 | 0.57 | 7.0 | HEPES | 50 | 50 | -1.200 | -2.800 |
| RDX[77] | Magnetite | 1.00 | 44 | 1.6 | 0.76 | 0.82 | 7.5 | HEPES | 50 | 50 | -0.490 | -2.100 |
| RDX[77] | Magnetite | 1.00 | 44 | 1.6 | 0.56 | 1.01 | 8.0 | HEPES | 50 | 50 | -0.590 | -2.200 |
| NG[78] | Magnetite | 4.00 | 0.56 | 4.0 | 7.4 | HEPES | 90 | 226 | ||||
| NG[79] | Pyrite | 20.00 | 0.53 | 7.4 | HEPES | 100 | 307 | -2.213 | -3.238 | |||
| TNT[79] | Pyrite | 20.00 | 0.53 | 7.4 | HEPES | 100 | 242 | -2.812 | -3.837 | |||
| RDX[79] | Pyrite | 20.00 | 0.53 | 7.4 | HEPES | 100 | 201 | -3.058 | -4.083 | |||
| RDX[51] | Carbonate Green Rust | 5.00 | 36 | 7.0 | 100 | |||||||
| RDX[51] | Sulfate Green Rust | 5.00 | 20 | 7.0 | 100 | |||||||
| DNAN[80] | Sulfate Green Rust | 10.00 | 8.4 | 500 | ||||||||
| NTO[80] | Sulfate Green Rust | 10.00 | 8.4 | 500 | ||||||||
| DNAN[81] | Magnetite | 2.00 | 17.8 | 1.0 | 7.0 | NaHCO3 | 10 | 200 | -0.100 | -1.700 | ||
| DNAN[81] | Mackinawite | 1.50 | 7.0 | NaHCO3 | 10 | 200 | 0.061 | |||||
| DNAN[81] | Goethite | 1.00 | 103.8 | 1.0 | 7.0 | NaHCO3 | 10 | 200 | 0.410 | -1.600 | ||
| RDX[82] | Magnetite | 0.62 | 1.0 | 7.0 | NaHCO3 | 10 | 17.5 | -1.100 | ||||
| RDX[82] | Magnetite | 0.62 | 7.0 | MOPS | 50 | 17.5 | -0.270 | |||||
| RDX[82] | Magnetite | 0.62 | 1.0 | 7.0 | MOPS | 10 | 17.6 | -0.480 | ||||
| NTO[83] | Hematite | 1.00 | 5.7 | 1.0 | 0.92 | 0.08 | 5.5 | MES | 50 | 30 | -0.550 | -1.308 |
| NTO[83] | Hematite | 1.00 | 5.7 | 1.0 | 0.85 | 0.15 | 6.0 | MES | 50 | 30 | 0.619 | -0.140 |
| NTO[83] | Hematite | 1.00 | 5.7 | 1.0 | 0.9 | 0.10 | 6.5 | MES | 50 | 30 | 1.348 | 0.590 |
| NTO[83] | Hematite | 1.00 | 5.7 | 1.0 | 0.77 | 0.23 | 7.0 | MOPS | 50 | 30 | 2.167 | 1.408 |
| NTO[83] | Hematite a | 1.00 | 5.7 | 1.01 | 5.5 | MES | 50 | 30 | -1.444 | -2.200 | ||
| NTO[83] | Hematite a | 1.00 | 5.7 | 0.97 | 6.0 | MES | 50 | 30 | -0.658 | -1.413 | ||
| NTO[83] | Hematite a | 1.00 | 5.7 | 0.87 | 6.5 | MES | 50 | 30 | 0.068 | -0.688 | ||
| NTO[83] | Hematite a | 1.00 | 5.7 | 0.79 | 7.0 | MOPS | 50 | 30 | 1.210 | 0.456 | ||
| RDX[50] | Mackinawite | 0.45 | 6.5 | NaHCO3 | 10 | 250 | -0.092 | |||||
| RDX[50] | Mackinawite | 0.45 | 7.0 | NaHCO3 | 10 | 250 | 0.009 | |||||
| RDX[50] | Mackinawite | 0.45 | 7.5 | NaHCO3 | 10 | 250 | 0.158 | |||||
| RDX[50] | Green Rust | 5 | 6.5 | NaHCO3 | 10 | 250 | -1.301 | |||||
| RDX[50] | Green Rust | 5 | 7.0 | NaHCO3 | 10 | 250 | -1.097 | |||||
| RDX[50] | Green Rust | 5 | 7.5 | NaHCO3 | 10 | 250 | -0.745 | |||||
| RDX[50] | Goethite | 0.5 | 1 | 1 | 6.5 | NaHCO3 | 10 | 250 | -0.921 | |||
| RDX[50] | Goethite | 0.5 | 1 | 1 | 7.0 | NaHCO3 | 10 | 250 | -0.347 | |||
| RDX[50] | Goethite | 0.5 | 1 | 1 | 7.5 | NaHCO3 | 10 | 250 | 0.009 | |||
| RDX[50] | Hematite | 0.5 | 1 | 1 | 6.5 | NaHCO3 | 10 | 250 | -0.824 | |||
| RDX[50] | Hematite | 0.5 | 1 | 1 | 7.0 | NaHCO3 | 10 | 250 | -0.456 | |||
| RDX[50] | Hematite | 0.5 | 1 | 1 | 7.5 | NaHCO3 | 10 | 250 | -0.237 | |||
| RDX[50] | Magnetite | 2 | 1 | 1 | 6.5 | NaHCO3 | 10 | 250 | -1.523 | |||
| RDX[50] | Magnetite | 2 | 1 | 1 | 7.0 | NaHCO3 | 10 | 250 | -0.824 | |||
| RDX[50] | Magnetite | 2 | 1 | 1 | 7.5 | NaHCO3 | 10 | 250 | -0.229 | |||
| DNAN[84] | Mackinawite | 4.28 | 0.25 | 6.5 | NaHCO3 | 8.5 + 20% CO2(g) | 400 | 0.836 | 0.806 | |||
| DNAN[84] | Mackinawite | 4.28 | 0.25 | 7.6 | NaHCO3 | 95.2 + 20% CO2(g) | 400 | 0.762 | 0.732 | |||
| DNAN[84] | Commercial FeS | 5.00 | 0.214 | 6.5 | NaHCO3 | 8.5 + 20% CO2(g) | 400 | 0.477 | 0.447 | |||
| DNAN[84] | Commercial FeS | 5.00 | 0.214 | 7.6 | NaHCO3 | 95.2 + 20% CO2(g) | 400 | 0.745 | 0.716 | |||
| NTO[84] | Mackinawite | 4.28 | 0.25 | 6.5 | NaHCO3 | 8.5 + 20% CO2(g) | 1000 | 0.663 | 0.633 | |||
| NTO[84] | Mackinawite | 4.28 | 0.25 | 7.6 | NaHCO3 | 95.2 + 20% CO2(g) | 1000 | 0.521 | 0.491 | |||
| NTO[84] | Commercial FeS | 5.00 | 0.214 | 6.5 | NaHCO3 | 8.5 + 20% CO2(g) | 1000 | 0.492 | 0.462 | |||
| NTO[84] | Commercial FeS | 5.00 | 0.214 | 7.6 | NaHCO3 | 95.2 + 20% CO2(g) | 1000 | 0.427 | 0.398 | |||
| Notes: a Dithionite-reduced hematite; experiments conducted in the presence of 1 mM sulfite. b Initial aqueous Fe(II); not added for Fe(II) bearing minerals. c Aqueous Fe(II) after 24h of equilibration. d Difference between b and c. e Initial nominal MC concentration. f Pseudo-first order rate constant. g Surface area normalized rate constant calculated as kObs / (surface area concentration) or kObs / (surface area × mineral loading). | ||||||||||||
| NAC a | Iron Oxide | Iron oxide loading (g/L) |
Surface area (m2/g) |
Fe(II)aq initial (mM) b |
Fe(II)aq after 24 h (mM) c |
Fe(II)aq sorbed (mM) d |
pH | Buffer | Buffer (mM) |
NAC initial (μM) e |
log kobs (h-1) f |
log kSA (Lh-1m-2) g |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NB[12] | Magnetite | 0.200 | 56.00 | 1.5000 | 7.00 | Phosphate | 10 | 50 | 1.05E+00 | 7.75E-04 | ||
| 4-ClNB[12] | Magnetite | 0.200 | 56.00 | 1.5000 | 7.00 | Phosphate | 10 | 50 | 1.14E+00 | 8.69E-02 | ||
| 4-ClNB[30] | Goethite | 0.640 | 17.50 | 1.5000 | 7.00 | MOPS | 25 | 50 | -1.01E-01 | -1.15E+00 | ||
| 4-ClNB[14] | Goethite | 1.500 | 16.20 | 1.2400 | 0.9600 | 0.2800 | 7.20 | MOPS | 1.2 | 0.5 - 3 | 1.68E+00 | 2.80E-01 |
| 4-ClNB[14] | Hematite | 1.800 | 13.70 | 1.0400 | 1.0100 | 0.0300 | 7.20 | MOPS | 1.2 | 0.5 - 3 | -2.32E+00 | -3.72E+00 |
| 4-ClNB[14] | Lepidocrocite | 1.400 | 17.60 | 1.1400 | 1.0000 | 0.1400 | 7.20 | MOPS | 1.2 | 0.5 - 3 | 1.51E+00 | 1.20E-01 |
| 4-CNNB[7] | Ferrihydrite | 0.004 | 292.00 | 0.3750 | 0.3500 | 0.0300 | 7.97 | HEPES | 25 | 15 | -7.47E-01 | -8.61E-01 |
| 4-CNNB[7] | Ferrihydrite | 0.004 | 292.00 | 0.3750 | 0.3700 | 0.0079 | 7.67 | HEPES | 25 | 15 | -1.51E+00 | -1.62E+00 |
| 4-CNNB[7] | Ferrihydrite | 0.004 | 292.00 | 0.3750 | 0.3600 | 0.0200 | 7.50 | MOPS | 25 | 15 | -2.15E+00 | -2.26E+00 |
| 4-CNNB[7] | Ferrihydrite | 0.004 | 292.00 | 0.3750 | 0.3600 | 0.0120 | 7.28 | MOPS | 25 | 15 | -3.08E+00 | -3.19E+00 |
| 4-CNNB[7] | Ferrihydrite | 0.004 | 292.00 | 0.3750 | 0.3700 | 0.0004 | 7.00 | MOPS | 25 | 15 | -3.22E+00 | -3.34E+00 |
| 4-CNNB[7] | Ferrihydrite | 0.004 | 292.00 | 0.3750 | 0.3700 | 0.0024 | 6.80 | MOPSO | 25 | 15 | -3.72E+00 | -3.83E+00 |
| 4-CNNB[7] | Ferrihydrite | 0.004 | 292.00 | 0.3750 | 0.3700 | 0.0031 | 6.60 | MES | 25 | 15 | -3.83E+00 | -3.94E+00 |
| 4-CNNB[7] | Ferrihydrite | 0.020 | 292.00 | 0.3750 | 0.3700 | 0.0031 | 6.60 | MES | 25 | 15 | -3.83E+00 | -4.60E+00 |
| 4-CNNB[7] | Ferrihydrite | 0.110 | 292.00 | 0.3750 | 0.3700 | 0.0032 | 6.60 | MES | 25 | 15 | -1.57E+00 | -3.08E+00 |
| 4-CNNB[7] | Ferrihydrite | 0.220 | 292.00 | 0.3750 | 0.3700 | 0.0040 | 6.60 | MES | 25 | 15 | -1.12E+00 | -2.93E+00 |
| 4-CNNB[7] | Ferrihydrite | 0.551 | 292.00 | 0.3750 | 0.3700 | 0.0092 | 6.60 | MES | 25 | 15 | -6.18E-01 | -2.82E+00 |
| 4-CNNB[7] | Ferrihydrite | 1.099 | 292.00 | 0.3750 | 0.3500 | 0.0240 | 6.60 | MES | 25 | 15 | -3.66E-01 | -2.87E+00 |
| 4-CNNB[7] | Ferrihydrite | 1.651 | 292.00 | 0.3750 | 0.3400 | 0.0340 | 6.60 | MES | 25 | 15 | -8.35E-02 | -2.77E+00 |
| 4-CNNB[7] | Ferrihydrite | 2.199 | 292.00 | 0.3750 | 0.3300 | 0.0430 | 6.60 | MES | 25 | 15 | -3.11E-02 | -2.84E+00 |
| 4-CNNB[7] | Hematite | 0.038 | 34.00 | 0.3750 | 0.3320 | 0.0430 | 7.97 | HEPES | 25 | 15 | 1.63E+00 | 1.52E+00 |
| 4-CNNB[7] | Hematite | 0.038 | 34.00 | 0.3750 | 0.3480 | 0.0270 | 7.67 | HEPES | 25 | 15 | 1.26E+00 | 1.15E+00 |
| 4-CNNB[7] | Hematite | 0.038 | 34.00 | 0.3750 | 0.3470 | 0.0280 | 7.50 | MOPS | 25 | 15 | 7.23E-01 | 6.10E-01 |
| 4-CNNB[7] | Hematite | 0.038 | 34.00 | 0.3750 | 0.3680 | 0.0066 | 7.28 | MOPS | 25 | 15 | 4.53E-02 | -6.86E-02 |
| 4-CNNB[7] | Hematite | 0.038 | 34.00 | 0.3750 | 0.3710 | 0.0043 | 7.00 | MOPS | 25 | 15 | -3.12E-01 | -4.26E-01 |
| 4-CNNB[7] | Hematite | 0.038 | 34.00 | 0.3750 | 0.3710 | 0.0042 | 6.80 | MOPSO | 25 | 15 | -7.75E-01 | -8.89E-01 |
| 4-CNNB[7] | Hematite | 0.038 | 34.00 | 0.3750 | 0.3680 | 0.0069 | 6.60 | MES | 25 | 15 | -1.39E+00 | -1.50E+00 |
| 4-CNNB[7] | Hematite | 0.038 | 34.00 | 0.3750 | 0.3750 | 0.0003 | 6.10 | MES | 25 | 15 | -2.77E+00 | -2.88E+00 |
| 4-CNNB[7] | Hematite | 0.016 | 34.00 | 0.3750 | 0.3730 | 0.0024 | 6.60 | MES | 25 | 15 | -3.20E+00 | -2.95E+00 |
| 4-CNNB[7] | Hematite | 0.024 | 34.00 | 0.3750 | 0.3690 | 0.0064 | 6.60 | MES | 25 | 15 | -2.74E+00 | -2.66E+00 |
| 4-CNNB[7] | Hematite | 0.033 | 34.00 | 0.3750 | 0.3680 | 0.0069 | 6.60 | MES | 25 | 15 | -1.39E+00 | -1.43E+00 |
| 4-CNNB[7] | Hematite | 0.177 | 34.00 | 0.3750 | 0.3640 | 0.0110 | 6.60 | MES | 25 | 15 | 3.58E-01 | -4.22E-01 |
| 4-CNNB[7] | Hematite | 0.353 | 34.00 | 0.3750 | 0.3630 | 0.0120 | 6.60 | MES | 25 | 15 | 9.97E-01 | -8.27E-02 |
| 4-CNNB[7] | Hematite | 0.885 | 34.00 | 0.3750 | 0.3480 | 0.0270 | 6.60 | MES | 25 | 15 | 1.34E+00 | -1.34E-01 |
| 4-CNNB[7] | Hematite | 1.771 | 34.00 | 0.3750 | 0.3380 | 0.0370 | 6.60 | MES | 25 | 15 | 1.78E+00 | 3.03E-04 |
| 4-CNNB[7] | Lepidocrocite | 0.027 | 49.00 | 0.3750 | 0.3460 | 0.0290 | 7.97 | HEPES | 25 | 15 | 1.31E+00 | 1.20E+00 |
| 4-CNNB[7] | Lepidocrocite | 0.027 | 49.00 | 0.3750 | 0.3610 | 0.0140 | 7.67 | HEPES | 25 | 15 | 5.82E-01 | 4.68E-01 |
| 4-CNNB[7] | Lepidocrocite | 0.027 | 49.00 | 0.3750 | 0.3480 | 0.0270 | 7.50 | MOPS | 25 | 15 | 4.92E-02 | -6.47E-02 |
| 4-CNNB[7] | Lepidocrocite | 0.027 | 49.00 | 0.3750 | 0.3640 | 0.0110 | 7.28 | MOPS | 25 | 15 | -3.77E-01 | -4.90E-01 |
| 4-CNNB[7] | Lepidocrocite | 0.027 | 49.00 | 0.3750 | 0.3640 | 0.0110 | 7.00 | MOPS | 25 | 15 | -1.25E+00 | -1.36E+00 |
| 4-CNNB[7] | Lepidocrocite | 0.027 | 49.00 | 0.3750 | 0.3620 | 0.0130 | 6.80 | MOPSO | 25 | 15 | -1.74E+00 | -1.86E+00 |
| 4-CNNB[7] | Lepidocrocite | 0.027 | 49.00 | 0.3750 | 0.3740 | 0.0015 | 6.60 | MES | 25 | 15 | -2.58E+00 | -2.69E+00 |
| 4-CNNB[7] | Lepidocrocite | 0.027 | 49.00 | 0.3750 | 0.3700 | 0.0046 | 6.10 | MES | 25 | 15 | -3.80E+00 | -3.92E+00 |
| 4-CNNB[7] | Lepidocrocite | 0.020 | 49.00 | 0.3750 | 0.3740 | 0.0014 | 6.60 | MES | 25 | 15 | -2.58E+00 | -2.57E+00 |
| 4-CNNB[7] | Lepidocrocite | 11.980 | 49.00 | 0.3750 | 0.3620 | 0.0130 | 6.60 | MES | 25 | 15 | -5.78E-01 | -3.35E+00 |
| 4-CNNB[7] | Lepidocrocite | 0.239 | 49.00 | 0.3750 | 0.3530 | 0.0220 | 6.60 | MES | 25 | 15 | -2.78E-02 | -1.10E+00 |
| 4-CNNB[7] | Lepidocrocite | 0.600 | 49.00 | 0.3750 | 0.3190 | 0.0560 | 6.60 | MES | 25 | 15 | 3.75E-01 | -1.09E+00 |
| 4-CNNB[7] | Lepidocrocite | 1.198 | 49.00 | 0.3750 | 0.2700 | 0.1050 | 6.60 | MES | 25 | 15 | 5.05E-01 | -1.26E+00 |
| 4-CNNB[7] | Lepidocrocite | 1.798 | 49.00 | 0.3750 | 0.2230 | 0.1520 | 6.60 | MES | 25 | 15 | 5.56E-01 | -1.39E+00 |
| 4-CNNB[7] | Lepidocrocite | 2.388 | 49.00 | 0.3750 | 0.1820 | 0.1930 | 6.60 | MES | 25 | 15 | 5.28E-01 | -1.54E+00 |
| 4-CNNB[7] | Goethite | 0.025 | 51.00 | 0.3750 | 0.3440 | 0.0310 | 7.97 | HEPES | 25 | 15 | 9.21E-01 | 8.07E-01 |
| 4-CNNB[7] | Goethite | 0.025 | 51.00 | 0.3750 | 0.3660 | 0.0094 | 7.67 | HEPES | 25 | 15 | 3.05E-01 | 1.91E-01 |
| 4-CNNB[7] | Goethite | 0.025 | 51.00 | 0.3750 | 0.3570 | 0.0180 | 7.50 | MOPS | 25 | 15 | -9.96E-02 | -2.14E-01 |
| 4-CNNB[7] | Goethite | 0.025 | 51.00 | 0.3750 | 0.3640 | 0.0110 | 7.28 | MOPS | 25 | 15 | -8.18E-01 | -9.32E-01 |
| 4-CNNB[7] | Goethite | 0.025 | 51.00 | 0.3750 | 0.3670 | 0.0084 | 7.00 | MOPS | 25 | 15 | -1.61E+00 | -1.73E+00 |
| 4-CNNB[7] | Goethite | 0.025 | 51.00 | 0.3750 | 0.3750 | 0.0004 | 6.80 | MOPSO | 25 | 15 | -1.82E+00 | -1.93E+00 |
| 4-CNNB[7] | Goethite | 0.025 | 51.00 | 0.3750 | 0.3730 | 0.0018 | 6.60 | MES | 25 | 15 | -2.26E+00 | -2.37E+00 |
| 4-CNNB[7] | Goethite | 0.025 | 51.00 | 0.3750 | 0.3670 | 0.0076 | 6.10 | MES | 25 | 15 | -3.56E+00 | -3.67E+00 |
| 4-CNNB[7] | Goethite | 0.020 | 51.00 | 0.3750 | 0.3680 | 0.0069 | 6.60 | MES | 25 | 15 | -2.26E+00 | -2.27E+00 |
| 4-CNNB[7] | Goethite | 0.110 | 51.00 | 0.3750 | 0.3660 | 0.0090 | 6.60 | MES | 25 | 15 | -3.19E-01 | -1.07E+00 |
| 4-CNNB[7] | Goethite | 0.220 | 51.00 | 0.3750 | 0.3540 | 0.0210 | 6.60 | MES | 25 | 15 | 5.00E-01 | -5.50E-01 |
| 4-CNNB[7] | Goethite | 0.551 | 51.00 | 0.3750 | 0.3220 | 0.0530 | 6.60 | MES | 25 | 15 | 1.03E+00 | -4.15E-01 |
| 4-CNNB[7] | Goethite | 1.100 | 51.00 | 0.3750 | 0.2740 | 0.1010 | 6.60 | MES | 25 | 15 | 1.46E+00 | -2.88E-01 |
| 4-CNNB[7] | Goethite | 1.651 | 51.00 | 0.3750 | 0.2330 | 0.1420 | 6.60 | MES | 25 | 15 | 1.66E+00 | -2.70E-01 |
| 4-CNNB[7] | Goethite | 2.196 | 51.00 | 0.3750 | 0.1910 | 0.1840 | 6.60 | MES | 25 | 15 | 1.83E+00 | -2.19E-01 |
| 4-CNNB[7] | Goethite | 0.142 | 51.00 | 0.3750 | 6.60 | MES | 25 | 15 | 1.99E-01 | -6.61E-01 | ||
| 4-AcNB[7] | Goethite | 0.142 | 51.00 | 0.3750 | 6.60 | MES | 25 | 15 | -6.85E-02 | -9.28E-01 | ||
| 4-ClNB[7] | Goethite | 0.142 | 51.00 | 0.3750 | 6.60 | MES | 25 | 15 | -5.47E-01 | -1.41E+00 | ||
| 4-BrNB[7] | Goethite | 0.142 | 51.00 | 0.3750 | 6.60 | MES | 25 | 15 | -5.73E-01 | -1.43E+00 | ||
| NB[7] | Goethite | 0.142 | 51.00 | 0.3750 | 6.60 | MES | 25 | 15 | -7.93E-01 | -1.65E+00 | ||
| 4-MeNB[7] | Goethite | 0.142 | 51.00 | 0.3750 | 6.60 | MES | 25 | 15 | -9.79E-01 | -1.84E+00 | ||
| 4-ClNB[11] | Goethite | 0.040 | 186.75 | 1.0000 | 0.8050 | 0.1950 | 7.00 | 5.53E-01 | -3.20E-01 | |||
| 4-ClNB[11] | Goethite | 7.516 | 16.10 | 1.0000 | 0.9260 | 0.0740 | 7.00 | 2.08E+00 | 0.00E+00 | |||
| 4-ClNB[11] | Ferrihydrite | 0.111 | 252.60 | 1.0000 | 0.6650 | 0.3350 | 7.00 | -1.12E-01 | -1.56E+00 | |||
| 4-ClNB[11] | Lepidocrocite | 2.384 | 60.40 | 1.0000 | 0.9250 | 0.0750 | 7.00 | 1.30E+00 | -8.60E-01 | |||
| 4-ClNB[10] | Goethite | 10.000 | 14.90 | 1.0000 | 7.20 | HEPES | 10 | 10 - 50 | 2.26E+00 | 8.00E-02 | ||
| 4-ClNB[10] | Goethite | 3.000 | 14.90 | 1.0000 | 7.20 | HEPES | 10 | 10 - 50 | 2.38E+00 | 7.30E-01 | ||
| 4-ClNB[10] | Lepidocrocite | 2.700 | 16.20 | 1.0000 | 7.20 | HEPES | 10 | 10 - 50 | 9.20E-01 | -7.20E-01 | ||
| 4-ClNB[10] | Lepidocrocite | 10.000 | 16.20 | 1.0000 | 7.20 | HEPES | 10 | 10 - 50 | 1.03E+00 | -1.18E+00 | ||
| 4-ClNB[13] | Goethite | 0.325 | 140.00 | 1.0000 | 7.00 | Bicarbonate | 10 | 100 | -1.32E-01 | -1.79E+00 | ||
| 4-ClNB[13] | Goethite | 0.325 | 140.00 | 1.0000 | 6.50 | Bicarbonate | 10 | 100 | -4.42E-01 | -2.10E+00 | ||
| NB[16] | Goethite | 0.500 | 30.70 | 0.1000 | 0.1120 | 0.0090 | 6.00 | MES | 25 | 12 | -1.42E+00 | -2.61E+00 |
| NB[16] | Goethite | 0.500 | 30.70 | 0.5000 | 0.5150 | 0.0240 | 6.00 | MES | 25 | 15 | -7.45E-01 | -1.93E+00 |
| NB[16] | Goethite | 0.500 | 30.70 | 1.0000 | 1.0280 | 0.0140 | 6.00 | MES | 25 | 19 | -7.45E-01 | -1.93E+00 |
| NB[16] | Goethite | 1.000 | 30.70 | 0.1000 | 0.0960 | 0.0260 | 6.00 | MES | 25 | 13 | -1.12E+00 | -2.61E+00 |
| NB[16] | Goethite | 1.000 | 30.70 | 0.5000 | 0.4890 | 0.0230 | 6.00 | MES | 25 | 14 | -5.53E-01 | -2.04E+00 |
| NB[16] | Goethite | 1.000 | 30.70 | 1.0000 | 0.9870 | 0.0380 | 6.00 | MES | 25 | 19 | -2.52E-01 | -1.74E+00 |
| NB[16] | Goethite | 2.000 | 30.70 | 0.1000 | 0.0800 | 0.0490 | 6.00 | MES | 25 | 11 | -8.86E-01 | -2.67E+00 |
| NB[16] | Goethite | 2.000 | 30.70 | 0.6000 | 0.4890 | 0.0640 | 6.00 | MES | 25 | 14 | -1.08E-01 | -1.90E+00 |
| NB[16] | Goethite | 2.000 | 30.70 | 1.1000 | 0.9870 | 0.0670 | 6.00 | MES | 25 | 14 | 2.30E-01 | -1.56E+00 |
| NB[16] | Goethite | 4.000 | 30.70 | 0.1000 | 0.0600 | 0.0650 | 6.00 | MES | 25 | 11 | -8.89E-01 | -2.98E+00 |
| NB[16] | Goethite | 4.000 | 30.70 | 0.6000 | 0.3960 | 0.1550 | 6.00 | MES | 25 | 17 | 1.43E-01 | -1.95E+00 |
| NB[16] | Goethite | 4.000 | 30.70 | 1.0000 | 0.8360 | 0.1450 | 6.00 | MES | 25 | 16 | 4.80E-01 | -1.61E+00 |
| NB[16] | Goethite | 4.000 | 30.70 | 5.6000 | 5.2110 | 0.3790 | 6.00 | MES | 25 | 15 | 1.17E+00 | -9.19E-01 |
| NB[16] | Goethite | 1.000 | 30.70 | 0.1000 | 0.0870 | 0.0300 | 6.50 | MES | 25 | 5.5 | -1.74E-01 | -1.66E+00 |
| NB[16] | Goethite | 1.000 | 30.70 | 0.5000 | 0.4920 | 0.0300 | 6.50 | MES | 25 | 15 | 3.64E-01 | -1.12E+00 |
| NB[16] | Goethite | 1.000 | 30.70 | 1.0000 | 0.9390 | 0.0650 | 6.50 | MES | 25 | 18 | 6.70E-01 | -8.17E-01 |
| NB[16] | Goethite | 2.000 | 30.70 | 0.1000 | 0.0490 | 0.0730 | 6.50 | MES | 25 | 5.2 | 3.01E-01 | -1.49E+00 |
| NB[16] | Goethite | 2.000 | 30.70 | 0.5000 | 0.4640 | 0.0710 | 6.50 | MES | 25 | 14 | 8.85E-01 | -9.03E-01 |
| NB[16] | Goethite | 2.000 | 30.70 | 1.0000 | 0.9130 | 0.1280 | 6.50 | MES | 25 | 16 | 1.12E+00 | -6.64E-01 |
| NB[16] | Goethite | 1.000 | 30.70 | 0.1000 | 0.0630 | 0.0480 | 7.00 | MOPS | 25 | 5.3 | 6.12E-01 | -8.75E-01 |
| NB[16] | Goethite | 1.000 | 30.70 | 0.5000 | 0.4690 | 0.0520 | 7.00 | MOPS | 25 | 9 | 1.51E+00 | 2.07E-02 |
| NB[16] | Goethite | 1.000 | 30.70 | 1.0000 | 0.9360 | 0.1090 | 7.00 | MOPS | 25 | 18 | 1.33E+00 | -1.53E-01 |
| NB[16] | Goethite | 2.000 | 30.70 | 0.1000 | 0.0290 | 0.0880 | 7.00 | MOPS | 25 | 12 | 6.85E-01 | -1.10E+00 |
| NB[16] | Goethite | 2.000 | 30.70 | 0.5000 | 0.3950 | 0.1450 | 7.00 | MOPS | 25 | 15 | 1.59E+00 | -1.95E-01 |
| Notes: a The NACs are Nitrobenzene (NB), 4-chloronitrobenzene(4-ClNB), 4-cyanonitrobenzene (4-CNNB), 4-acetylnitrobenzene (4-AcNB), 4-bromonitrobenzene (4-BrNB), 4-nitrotoluene (4-MeNB). b Initial aqueous Fe(II). c Aqueous Fe(II) after 24h of equilibration. d Difference between b and c. e Initial nominal NAC concentration. f Pseudo-first order rate constant. g Surface area normalized rate constant calculated as kObs / (surface area × mineral loading). | ||||||||||||
Iron(II) can be complexed by a myriad of organic ligands and may thereby become more reactive towards MCs and other pollutants. The reactivity of an Fe(II)-organic complex depends on the relative preference of the organic ligand for Fe(III) versus Fe(II)[5]. Since the majority of naturally occurring ligands complex Fe(III) more strongly than Fe(II), the reduction potential of the resulting Fe(III) complex is lower than that of aqueous Fe(III); therefore, complexation by organic ligands often renders Fe(II) a stronger reductant thermodynamically[85]. The reactivity of dissolved Fe(II)-organic complexes towards NACs/MCs has been investigated. The intrinsic, second-order rate constants and one electron reduction potentials are listed in Table 2.
In addition to forming organic complexes, iron is ubiquitous in minerals. Iron-bearing minerals play an important role in controlling the environmental fate of contaminants through adsorption[86][87] and reduction[88] processes. Studies have shown that aqueous Fe(II) itself cannot reduce NACs/MCs at circumneutral pH[12][77] but in the presence of an iron oxide (e.g., goethite, hematite, lepidocrocite, ferrihydrite, or magnetite), NACs[7][12][13][14][21] and MCs such as TNT[30], RDX[77], DNAN[81], and NG[78] can be rapidly reduced. Unlike ferric oxides, Fe(II)-bearing minerals including clays[21][22][23][24][25][26][27], green rust[51][80], mackinawite[14][81][84] and pyrite[14][79] do not need aqueous Fe(II) to be reactive toward NACs/MCs. However, upon oxidation, sulfate green rust was converted into lepidocrocite[80], and mackinawite into goethite[84], suggesting that aqueous Fe(II) coupled to Fe(III) oxides might be at least partially responsible for continued degradation of NACs/MCs in the subsurface once the parent reductant (e.g., green rust or iron sulfide) oxidizes.
The reaction conditions and rate constants for a list of studies on MC reduction by iron oxide-aqueous Fe(II) redox couples and by other Fe(II)-containing minerals are shown in Table 3[30][51][77][81][79][82][83]. Unlike hydroquinones and Fe(II) complexes, where second-order rate constants can be readily calculated, the reduction rate constants of NACs/MCs in mineral suspensions are often specific to the experimental conditions used and are usually reported as BET surface area-normalized reduction rate constants (kSA). In the case of iron oxide-Fe(II) redox couples, reduction rate constants have been shown to increase with pH (specifically, with [OH– ]2) and aqueous Fe(II) concentration, both of which correspond to a decrease in the system's reduction potential[7][9][83].
For minerals that contain structural iron(II) and can reduce pollutants in the absence of aqueous Fe(II), the observed rates of reduction increased with increasing structural Fe(II) content, as seen with iron-bearing clays[23][24] and green rust[51]. This dependency on Fe(II) content allows for the derivation of second-order rate constants, as shown on Table 3 for the reduction of RDX by green rust[51], and the development of reduction potential (EH)-based models[23][89][90][91], where EH represents the reduction potential of the iron-bearing clays. Iron-bearing expandable clay minerals represent a special case, which in addition to reduction can remove NACs/MCs through adsorption. This is particularly important for planar NACs/MCs that contain multiple electron-withdrawing nitro groups and can form strong electron donor-acceptor (EDA) complexes with the clay surface[21][25][26].
Although the second-order rate constants derived for Fe(II)-bearing minerals may allow comparison among different studies, they may not reflect changes in reactivity due to variations in surface area, pH, and the presence of ions. Anions such as bicarbonate[51][82][92] and phosphate[51][93] are known to decrease the reactivity of iron oxides-Fe(II) redox couples and green rust. Sulfite has also been shown to decrease the reactivity of hematite-Fe(II) towards the deprotonated form of NTO (Table 3)[83]. Exchanging cations in iron-bearing clays can change the reactivity of these minerals by up to 7-fold[21]. Thus, more comprehensive models are needed to account for the complexities in the subsurface environment.
The reduction of NACs has been widely studied in the presence of different iron minerals, pH, and Fe(II)(aq) concentrations (Table 4)[7][12][13][14][21]. Only selected NACs are included in Table 4. For more information on other NACs and ferruginous reductants, please refer to the cited references.
References
- ^ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Schwarzenbach, R.P., Gschwend, P.M., and Imboden, D.M., 2016. Environmental Organic Chemistry, 3rd Edition. John Wiley and Sons, Ltd, 1024 pages. ISBN: 978-1-118-76723-8
- ^ 2.0 2.1 Naka, D., Kim, D., and Strathmann, T.J., 2006. Abiotic Reduction of Nitroaromatic Compounds by Aqueous Iron(II)−Catechol Complexes. Environmental Science and Technology 40(9), pp. 3006–3012. DOI: 10.1021/es060044t
- ^ 3.0 3.1 3.2 Naka, D., Kim, D., Carbonaro, R.F., and Strathmann, T.J., 2008. Abiotic reduction of nitroaromatic contaminants by iron(II) complexes with organothiol ligands. Environmental Toxicology and Chemistry, 27(6), pp. 1257–1266. DOI: 10.1897/07-505.1
- ^ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 Hartenbach, A.E., Hofstetter, T.B., Aeschbacher, M., Sander, M., Kim, D., Strathmann, T.J., Arnold, W.A., Cramer, C.J., and Schwarzenbach, R.P., 2008. Variability of Nitrogen Isotope Fractionation during the Reduction of Nitroaromatic Compounds with Dissolved Reductants. Environmental Science and Technology 42(22), pp. 8352–8359. DOI: 10.1021/es801063u
- ^ 5.0 5.1 5.2 5.3 Kim, D., Duckworth, O.W., and Strathmann, T.J., 2009. Hydroxamate siderophore-promoted reactions between iron(II) and nitroaromatic groundwater contaminants. Geochimica et Cosmochimica Acta, 73(5), pp. 1297–1311. DOI: 10.1016/j.gca.2008.11.039
- ^ 6.0 6.1 6.2 Kim, D., and Strathmann, T.J., 2007. Role of Organically Complexed Iron(II) Species in the Reductive Transformation of RDX in Anoxic Environments. Environmental Science and Technology, 41(4), pp. 1257–1264. DOI: 10.1021/es062365a
- ^ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 7.14 7.15 7.16 7.17 7.18 7.19 7.20 7.21 7.22 7.23 7.24 7.25 7.26 7.27 7.28 7.29 7.30 7.31 7.32 7.33 7.34 7.35 7.36 7.37 7.38 7.39 7.40 7.41 7.42 7.43 7.44 7.45 7.46 7.47 7.48 7.49 7.50 7.51 7.52 7.53 7.54 7.55 7.56 7.57 7.58 7.59 7.60 7.61 7.62 7.63 7.64 7.65 7.66 7.67 7.68 Colón, D., Weber, E.J., and Anderson, J.L., 2006. QSAR Study of the Reduction of Nitroaromatics by Fe(II) Species. Environmental Science and Technology, 40(16), pp. 4976–4982. DOI: 10.1021/es052425x
- ^ Luan, F., Xie, L., Li, J., and Zhou, Q., 2013. Abiotic reduction of nitroaromatic compounds by Fe(II) associated with iron oxides and humic acid. Chemosphere, 91(7), pp. 1035–1041. DOI: 10.1016/j.chemosphere.2013.01.070
- ^ 9.0 9.1 Gorski, C.A., Edwards, R., Sander, M., Hofstetter, T.B., and Stewart, S.M., 2016. Thermodynamic Characterization of Iron Oxide–Aqueous Fe2+ Redox Couples. Environmental Science and Technology, 50(16), pp. 8538–8547. DOI: 10.1021/acs.est.6b02661
- ^ 10.0 10.1 10.2 10.3 10.4 Fan, D., Bradley, M.J., Hinkle, A.W., Johnson, R.L., and Tratnyek, P.G., 2016. Chemical Reactivity Probes for Assessing Abiotic Natural Attenuation by Reducing Iron Minerals. Environmental Science and Technology, 50(4), pp. 1868–1876. DOI: 10.1021/acs.est.5b05800
- ^ 11.0 11.1 11.2 11.3 11.4 Jones, A.M., Kinsela, A.S., Collins, R.N., and Waite, T.D., 2016. The reduction of 4-chloronitrobenzene by Fe(II)-Fe(III) oxide systems - correlations with reduction potential and inhibition by silicate. Journal of Hazardous Materials, 320, pp. 143–149. DOI: 10.1016/j.jhazmat.2016.08.031
- ^ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 Klausen, J., Troeber, S.P., Haderlein, S.B., and Schwarzenbach, R.P., 1995. Reduction of Substituted Nitrobenzenes by Fe(II) in Aqueous Mineral Suspensions. Environmental Science and Technology, 29(9), pp. 2396–2404. DOI: 10.1021/es00009a036
- ^ 13.0 13.1 13.2 13.3 13.4 Strehlau, J.H., Stemig, M.S., Penn, R.L., and Arnold, W.A., 2016. Facet-Dependent Oxidative Goethite Growth As a Function of Aqueous Solution Conditions. Environmental Science and Technology, 50(19), pp. 10406–10412. DOI: 10.1021/acs.est.6b02436
- ^ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 14.7 14.8 Elsner, M., Schwarzenbach, R.P., and Haderlein, S.B., 2004. Reactivity of Fe(II)-Bearing Minerals toward Reductive Transformation of Organic Contaminants. Environmental Science and Technology, 38(3), pp. 799–807. DOI: 10.1021/es0345569
- ^ Colón, D., Weber, E.J., and Anderson, J.L., 2008. Effect of Natural Organic Matter on the Reduction of Nitroaromatics by Fe(II) Species. Environmental Science and Technology, 42(17), pp. 6538–6543. DOI: 10.1021/es8004249
- ^ 16.00 16.01 16.02 16.03 16.04 16.05 16.06 16.07 16.08 16.09 16.10 16.11 16.12 16.13 16.14 16.15 16.16 16.17 16.18 16.19 16.20 16.21 16.22 16.23 16.24 Stewart, S.M., Hofstetter, T.B., Joshi, P. and Gorski, C.A., 2018. Linking Thermodynamics to Pollutant Reduction Kinetics by Fe2+ Bound to Iron Oxides. Environmental Science and Technology, 52(10), pp. 5600–5609. DOI: 10.1021/acs.est.8b00481 Article pdf
- ^ Klupinski, T.P., Chin, Y.P., and Traina, S.J., 2004. Abiotic Degradation of Pentachloronitrobenzene by Fe(II): Reactions on Goethite and Iron Oxide Nanoparticles. Environmental Science and Technology, 38(16), pp. 4353–4360. DOI: 10.1021/es035434j
- ^ Heijman, C.G., Holliger, C., Glaus, M.A., Schwarzenbach, R.P., and Zeyer, J., 1993. Abiotic Reduction of 4-Chloronitrobenzene to 4-Chloroaniline in a Dissimilatory Iron-Reducing Enrichment Culture. Applied and Environmental Microbiology, 59(12), pp. 4350–4353. DOI: 10.1128/aem.59.12.4350-4353.1993 Article pdf
- ^ Gorski, C.A., and Scherer, M.M., 2009. Influence of Magnetite Stoichiometry on FeII Uptake and Nitrobenzene Reduction. Environmental Science and Technology, 43(10), pp. 3675–3680. DOI: 10.1021/es803613a
- ^ Gorski, C.A., Nurmi, J.T., Tratnyek, P.G., Hofstetter, T.B. and Scherer, M.M., 2010. Redox Behavior of Magnetite: Implications for Contaminant Reduction. Environmental Science and Technology, 44(1), pp. 55–60. DOI: 10.1021/es9016848
- ^ 21.0 21.1 21.2 21.3 21.4 21.5 Hofstetter, T.B., Neumann, A., and Schwarzenbach, R.P., 2006. Reduction of Nitroaromatic Compounds by Fe(II) Species Associated with Iron-Rich Smectites. Environmental Science and Technology, 40(1), pp. 235–242. DOI: 10.1021/es0515147
- ^ 22.0 22.1 Schultz, C. A., and Grundl, T.J., 2000. pH Dependence on Reduction Rate of 4-Cl-Nitrobenzene by Fe(II)/Montmorillonite Systems. Environmental Science and Technology 34(17), pp. 3641–3648. DOI: 10.1021/es990931e
- ^ 23.0 23.1 23.2 23.3 Luan, F., Gorski, C.A., and Burgos, W.D., 2015. Linear Free Energy Relationships for the Biotic and Abiotic Reduction of Nitroaromatic Compounds. Environmental Science and Technology, 49(6), pp. 3557–3565. DOI: 10.1021/es5060918
- ^ 24.0 24.1 24.2 Luan, F., Liu, Y., Griffin, A.M., Gorski, C.A. and Burgos, W.D., 2015. Iron(III)-Bearing Clay Minerals Enhance Bioreduction of Nitrobenzene by Shewanella putrefaciens CN32. Environmental Science and Technology, 49(3), pp. 1418–1426. DOI: 10.1021/es504149y
- ^ 25.0 25.1 25.2 Hofstetter, T.B., Schwarzenbach, R.P. and Haderlein, S.B., 2003. Reactivity of Fe(II) Species Associated with Clay Minerals. Environmental Science and Technology, 37(3), pp. 519–528. DOI: 10.1021/es025955r
- ^ 26.0 26.1 26.2 Neumann, A., Hofstetter, T.B., Lüssi, M., Cirpka, O.A., Petit, S., and Schwarzenbach, R.P., 2008. Assessing the Redox Reactivity of Structural Iron in Smectites Using Nitroaromatic Compounds As Kinetic Probes. Environmental Science and Technology, 42(22), pp. 8381–8387. DOI: 10.1021/es801840x
- ^ 27.0 27.1 Hofstetter, T.B., Neumann, A., Arnold, W.A., Hartenbach, A.E., Bolotin, J., Cramer, C.J., and Schwarzenbach, R.P., 2008. Substituent Effects on Nitrogen Isotope Fractionation During Abiotic Reduction of Nitroaromatic Compounds. Environmental Science and Technology, 42(6), pp. 1997–2003. DOI: 10.1021/es702471k
- ^ 28.00 28.01 28.02 28.03 28.04 28.05 28.06 28.07 28.08 28.09 28.10 28.11 28.12 28.13 28.14 28.15 28.16 28.17 28.18 28.19 28.20 28.21 28.22 28.23 28.24 28.25 28.26 28.27 28.28 28.29 28.30 28.31 28.32 28.33 28.34 28.35 28.36 28.37 28.38 28.39 28.40 28.41 28.42 28.43 28.44 28.45 28.46 28.47 28.48 28.49 28.50 28.51 28.52 28.53 28.54 28.55 28.56 28.57 Schwarzenbach, R.P., Stierli, R., Lanz, K., and Zeyer, J., 1990. Quinone and Iron Porphyrin Mediated Reduction of Nitroaromatic Compounds in Homogeneous Aqueous Solution. Environmental Science and Technology, 24(10), pp. 1566–1574. DOI: 10.1021/es00080a017
- ^ Tratnyek, P.G., and Macalady, D.L., 1989. Abiotic Reduction of Nitro Aromatic Pesticides in Anaerobic Laboratory Systems. Journal of Agricultural and Food Chemistry, 37(1), pp. 248–254. DOI: 10.1021/jf00085a058
- ^ 30.00 30.01 30.02 30.03 30.04 30.05 30.06 30.07 30.08 30.09 30.10 30.11 30.12 30.13 30.14 30.15 30.16 30.17 30.18 30.19 30.20 Hofstetter, T.B., Heijman, C.G., Haderlein, S.B., Holliger, C. and Schwarzenbach, R.P., 1999. Complete Reduction of TNT and Other (Poly)nitroaromatic Compounds under Iron-Reducing Subsurface Conditions. Environmental Science and Technology, 33(9), pp. 1479–1487. DOI: 10.1021/es9809760
- ^ 31.00 31.01 31.02 31.03 31.04 31.05 31.06 31.07 31.08 31.09 31.10 31.11 31.12 31.13 31.14 31.15 31.16 31.17 Murillo-Gelvez, J., Hickey, K.P., Di Toro, D.M., Allen, H.E., Carbonaro, R.F., and Chiu, P.C., 2019. Experimental Validation of Hydrogen Atom Transfer Gibbs Free Energy as a Predictor of Nitroaromatic Reduction Rate Constants. Environmental Science and Technology, 53(10), pp. 5816–5827. DOI: 10.1021/acs.est.9b00910
- ^ Niedźwiecka, J.B., Drew, S.R., Schlautman, M.A., Millerick, K.A., Grubbs, E., Tharayil, N. and Finneran, K.T., 2017. Iron and Electron Shuttle Mediated (Bio)degradation of 2,4-Dinitroanisole (DNAN). Environmental Science and Technology, 51(18), pp. 10729–10735. DOI: 10.1021/acs.est.7b02433
- ^ 33.0 33.1 Kwon, M.J., and Finneran, K.T., 2006. Microbially Mediated Biodegradation of Hexahydro-1,3,5-Trinitro-1,3,5- Triazine by Extracellular Electron Shuttling Compounds. Applied and Environmental Microbiology, 72(9), pp. 5933–5941. DOI: 10.1128/AEM.00660-06 Article pdf
- ^ 34.0 34.1 34.2 Dunnivant, F.M., Schwarzenbach, R.P., and Macalady, D.L., 1992. Reduction of Substituted Nitrobenzenes in Aqueous Solutions Containing Natural Organic Matter. Environmental Science and Technology, 26(11), pp. 2133–2141. DOI: 10.1021/es00035a010
- ^ 35.0 35.1 Luan, F., Burgos, W.D., Xie, L., and Zhou, Q., 2010. Bioreduction of Nitrobenzene, Natural Organic Matter, and Hematite by Shewanella putrefaciens CN32. Environmental Science and Technology, 44(1), pp. 184–190. DOI: 10.1021/es901585z
- ^ 36.0 36.1 36.2 36.3 Murillo-Gelvez, J., di Toro, D.M., Allen, H.E., Carbonaro, R.F., and Chiu, P.C., 2021. Reductive Transformation of 3-Nitro-1,2,4-triazol-5-one (NTO) by Leonardite Humic Acid and Anthraquinone-2,6-disulfonate (AQDS). Environmental Science and Technology, 55(19), pp. 12973–12983. DOI: 10.1021/acs.est.1c03333
- ^ 37.0 37.1 Oh, S.-Y., Son, J.G., and Chiu, P.C., 2013. Biochar-Mediated Reductive Transformation of Nitro Herbicides and Explosives. Environmental Toxicology and Chemistry, 32(3), pp. 501–508. DOI: 10.1002/etc.2087 Article pdf
- ^ 38.0 38.1 Oh, S.-Y., and Chiu, P.C., 2009. Graphite- and Soot-Mediated Reduction of 2,4-Dinitrotoluene and Hexahydro-1,3,5-trinitro-1,3,5-triazine. Environmental Science & Technology, 43(18), pp. 6983–6988. DOI: 10.1021/es901433m
- ^ 39.0 39.1 Xu, W., Pignatello, J.J., and Mitch, W.A., 2015. Reduction of Nitroaromatics Sorbed to Black Carbon by Direct Reaction with Sorbed Sulfides. Environmental Science and Technology, 49(6), pp. 3419–3426. DOI: 10.1021/es5045198
- ^ Oh, S.-Y., Cha, D.K., and Chiu, P.C., 2002. Graphite-Mediated Reduction of 2,4-Dinitrotoluene with Elemental Iron. Environmental Science and Technology, 36(10), pp. 2178–2184. DOI: 10.1021/es011474g
- ^ Amezquita-Garcia, H.J., Razo-Flores, E., Cervantes, F.J., and Rangel-Mendez, J.R., 2013. Activated carbon fibers as redox mediators for the increased reduction of nitroaromatics. Carbon, 55, pp. 276–284. DOI: 10.1016/j.carbon.2012.12.062
- ^ 42.0 42.1 Xin, D., Girón, J., Fuller, M.E., and Chiu, P.C., 2022. Abiotic Reduction of 3-Nitro-1,2,4-triazol-5-one (NTO) and Other Munitions Constituents by Wood-Derived Biochar through Its Rechargeable Electron Storage Capacity. Environmental Science: Processes and Impacts, 24(2), pp. 316-329. DOI: 10.1039/D1EM00447F
- ^ Hojo, M., Takagi, Y. and Ogata, Y., 1960. Kinetics of the Reduction of Nitrobenzenes by Sodium Disulfide. Journal of the American Chemical Society, 82(10), pp. 2459–2462. DOI: 10.1021/ja01495a017
- ^ Zeng, T., Chin, Y.P., and Arnold, W.A., 2012. Potential for Abiotic Reduction of Pesticides in Prairie Pothole Porewaters. Environmental Science and Technology, 46(6), pp. 3177–3187. DOI: 10.1021/es203584d
- ^ US EPA, 2012. A Citizen’s Guide to Monitored Natural Attenuation. EPA document 542-F-12-014. Article pdf
- ^ Spain, J.C., Hughes, J.B., and Knackmuss, H.J., 2000. Biodegradation of Nitroaromatic Compounds and Explosives. CRC Press, 456 pages. ISBN: 9780367398491
- ^ Harris, N.J., and Lammertsma, K., 1996. Tautomerism, Ionization, and Bond Dissociations of 5-Nitro-2,4-dihydro-3H-1,2,4-triazolone. Journal of the American Chemical Society, 118(34), pp. 8048–8055. DOI: 10.1021/ja960834a
- ^ 48.0 48.1 Kwon, M.J., and Finneran, K.T., 2008. Biotransformation products and mineralization potential for hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) in abiotic versus biological degradation pathways with anthraquinone-2,6-disulfonate (AQDS) and Geobacter metallireducens. Biodegradation, 19(5), pp. 705–715. DOI: 10.1007/s10532-008-9175-5
- ^ 49.0 49.1 Halasz, A., and Hawari, J., 2011. Degradation Routes of RDX in Various Redox Systems. Aquatic Redox Chemistry, American Chemical Society, 1071(20), pp. 441-462. DOI: 10.1021/bk-2011-1071.ch020
- ^ 50.00 50.01 50.02 50.03 50.04 50.05 50.06 50.07 50.08 50.09 50.10 50.11 50.12 50.13 50.14 50.15 50.16 Tong, Y., Berens, M.J., Ulrich, B.A., Bolotin, J., Strehlau, J.H., Hofstetter, T.B., and Arnold, W.A., 2021. Exploring the Utility of Compound-Specific Isotope Analysis for Assessing Ferrous Iron-Mediated Reduction of RDX in the Subsurface. Environmental Science and Technology, 55(10), pp. 6752–6763. DOI: 10.1021/acs.est.0c08420
- ^ 51.0 51.1 51.2 51.3 51.4 51.5 51.6 51.7 51.8 Larese-Casanova, P., and Scherer, M.M., 2008. Abiotic Transformation of Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by Green Rusts. Environmental Science and Technology, 42(11), pp. 3975–3981. DOI: 10.1021/es702390b
- ^ Borch, T., Kretzschmar, R., Kappler, A., Cappellen, P.V., Ginder-Vogel, M., Voegelin, A., and Campbell, K., 2010. Biogeochemical Redox Processes and their Impact on Contaminant Dynamics. Environmental Science and Technology, 44(1), pp. 15–23. DOI: 10.1021/es9026248 Article pdf
- ^ Zhang, H., and Weber, E.J., 2009. Elucidating the Role of Electron Shuttles in Reductive Transformations in Anaerobic Sediments. Environmental Science and Technology, 43(4), pp. 1042–1048. DOI: 10.1021/es8017072
- ^ Schumacher, B.A., 2002. Methods for the Determination of Total Organic Carbon (TOC) in Soils and Sediments. U.S. EPA, Ecological Risk Assessment Support Center. Article pdf.
- ^ Schmidt, M.W.I., and Noack, A.G., 2000. Black carbon in soils and sediments: Analysis, distribution, implications, and current challenges. Global Biogeochemical Cycles, 14(3), pp. 777–793. DOI: 10.1029/1999GB001208 Article pdf
- ^ 56.0 56.1 Klüpfel, L., Keiluweit, M., Kleber, M., and Sander, M., 2014. Redox Properties of Plant Biomass-Derived Black Carbon (Biochar). Environmental Science and Technology, 48(10), pp. 5601–5611. DOI: 10.1021/es500906d
- ^ Xin, D., Xian, M., and Chiu, P.C., 2019. New methods for assessing electron storage capacity and redox reversibility of biochar. Chemosphere, 215, 827–834. DOI: 10.1016/j.chemosphere.2018.10.080
- ^ Saquing, J.M., Yu, Y.-H., and Chiu, P.C., 2016. Wood-Derived Black Carbon (Biochar) as a Microbial Electron Donor and Acceptor. Environmental Science and Technology Letters, 3(2), pp. 62–66. DOI: 10.1021/acs.estlett.5b00354
- ^ Sparks, D.L., 2003. Environmental Soil Chemistry, 2nd Edition. Elsevier Science and Technology Books. DOI: 10.1016/B978-0-12-656446-4.X5000-2
- ^ Scott, D.T., McKnight, D.M., Blunt-Harris, E.L., Kolesar, S.E., and Lovley, D.R., 1998. Quinone Moieties Act as Electron Acceptors in the Reduction of Humic Substances by Humics-Reducing Microorganisms. Environmental Science and Technology, 32(19), pp. 2984–2989. DOI: 10.1021/es980272q
- ^ Cory, R.M., and McKnight, D.M., 2005. Fluorescence Spectroscopy Reveals Ubiquitous Presence of Oxidized and Reduced Quinones in Dissolved Organic Matter. Environmental Science & Technology, 39(21), pp 8142–8149. DOI: 10.1021/es0506962
- ^ Fimmen, R.L., Cory, R.M., Chin, Y.P., Trouts, T.D., and McKnight, D.M., 2007. Probing the oxidation–reduction properties of terrestrially and microbially derived dissolved organic matter. Geochimica et Cosmochimica Acta, 71(12), pp. 3003–3015. DOI: 10.1016/j.gca.2007.04.009
- ^ Struyk, Z., and Sposito, G., 2001. Redox properties of standard humic acids. Geoderma, 102(3-4), pp. 329–346. DOI: 10.1016/S0016-7061(01)00040-4
- ^ Ratasuk, N., and Nanny, M.A., 2007. Characterization and Quantification of Reversible Redox Sites in Humic Substances. Environmental Science and Technology, 41(22), pp. 7844–7850. DOI: 10.1021/es071389u
- ^ Aeschbacher, M., Sander, M., and Schwarzenbach, R.P., 2010. Novel Electrochemical Approach to Assess the Redox Properties of Humic Substances. Environmental Science and Technology, 44(1), pp. 87–93. DOI: 10.1021/es902627p
- ^ Aeschbacher, M., Vergari, D., Schwarzenbach, R.P., and Sander, M., 2011. Electrochemical Analysis of Proton and Electron Transfer Equilibria of the Reducible Moieties in Humic Acids. Environmental Science and Technology, 45(19), pp. 8385–8394. DOI: 10.1021/es201981g
- ^ Bauer, I., and Kappler, A., 2009. Rates and Extent of Reduction of Fe(III) Compounds and O2 by Humic Substances. Environmental Science and Technology, 43(13), pp. 4902–4908. DOI: 10.1021/es900179s
- ^ Maurer, F., Christl, I. and Kretzschmar, R., 2010. Reduction and Reoxidation of Humic Acid: Influence on Spectroscopic Properties and Proton Binding. Environmental Science and Technology, 44(15), pp. 5787–5792. DOI: 10.1021/es100594t
- ^ Walpen, N., Schroth, M.H., and Sander, M., 2016. Quantification of Phenolic Antioxidant Moieties in Dissolved Organic Matter by Flow-Injection Analysis with Electrochemical Detection. Environmental Science and Technology, 50(12), pp. 6423–6432. DOI: 10.1021/acs.est.6b01120 Article pdf.
- ^ Aeschbacher, M., Graf, C., Schwarzenbach, R.P., and Sander, M., 2012. Antioxidant Properties of Humic Substances. Environmental Science and Technology, 46(9), pp. 4916–4925. DOI: 10.1021/es300039h
- ^ Nurmi, J.T., and Tratnyek, P.G., 2002. Electrochemical Properties of Natural Organic Matter (NOM), Fractions of NOM, and Model Biogeochemical Electron Shuttles. Environmental Science and Technology, 36(4), pp. 617–624. DOI: 10.1021/es0110731
- ^ Xin, D., 2021. Understanding the Electron Storage Capacity of Pyrogenic Black Carbon: Origin, Redox Reversibility, Spatial Distribution, and Environmental Applications. Doctoral Thesis, University of Delaware. Thesis pdf.
- ^ Riefler, R.G., and Smets, B.F., 2000. Enzymatic Reduction of 2,4,6-Trinitrotoluene and Related Nitroarenes: Kinetics Linked to One-Electron Redox Potentials. Environmental Science and Technology, 34(18), pp. 3900–3906. DOI: 10.1021/es991422f
- ^ 74.00 74.01 74.02 74.03 74.04 74.05 74.06 74.07 74.08 74.09 74.10 74.11 Salter-Blanc, A.J., Bylaska, E.J., Johnston, H.J., and Tratnyek, P.G., 2015. Predicting Reduction Rates of Energetic Nitroaromatic Compounds Using Calculated One-Electron Reduction Potentials. Environmental Science and Technology, 49(6), pp. 3778–3786. DOI: 10.1021/es505092s Article pdf.
- ^ 75.00 75.01 75.02 75.03 75.04 75.05 75.06 75.07 75.08 75.09 75.10 Gao, Y., Zhong, S., Torralba-Sanchez, T.L., Tratnyek, P.G., Weber, E.J., Chen, Y., and Zhang, H., 2021. Quantitative structure activity relationships (QSARs) and machine learning models for abiotic reduction of organic compounds by an aqueous Fe(II) complex. Water Research, 192, p. 116843. DOI: 10.1016/j.watres.2021.116843
- ^ 76.0 76.1 76.2 76.3 Uchimiya, M., Gorb, L., Isayev, O., Qasim, M.M., and Leszczynski, J., 2010. One-electron standard reduction potentials of nitroaromatic and cyclic nitramine explosives. Environmental Pollution, 158(10), pp. 3048–3053. DOI: 10.1016/j.envpol.2010.06.033
- ^ 77.00 77.01 77.02 77.03 77.04 77.05 77.06 77.07 77.08 77.09 77.10 77.11 77.12 77.13 77.14 77.15 Gregory, K.B., Larese-Casanova, P., Parkin, G.F., and Scherer, M.M., 2004. Abiotic Transformation of Hexahydro-1,3,5-trinitro-1,3,5-triazine by FeII Bound to Magnetite. Environmental Science and Technology, 38(5), pp. 1408–1414. DOI: 10.1021/es034588w
- ^ 78.0 78.1 Oh, S.-Y., Cha, D.K., Kim, B.J., and Chiu, P.C., 2004. Reduction of Nitroglycerin with Elemental Iron: Pathway, Kinetics, and Mechanisms. Environmental Science and Technology, 38(13), pp. 3723–3730. DOI: 10.1021/es0354667
- ^ 79.0 79.1 79.2 79.3 79.4 Oh, S.-Y., Chiu, P.C., and Cha, D.K., 2008. Reductive transformation of 2,4,6-trinitrotoluene, hexahydro-1,3,5-trinitro-1,3,5-triazine, and nitroglycerin by pyrite and magnetite. Journal of hazardous materials, 158(2-3), pp. 652–655. DOI: 10.1016/j.jhazmat.2008.01.078
- ^ 80.0 80.1 80.2 80.3 Khatiwada, R., Root, R.A., Abrell, L., Sierra-Alvarez, R., Field, J.A., and Chorover, J., 2018. Abiotic reduction of insensitive munition compounds by sulfate green rust. Environmental Chemistry, 15(5), pp. 259–266. DOI: 10.1071/EN17221
- ^ 81.0 81.1 81.2 81.3 81.4 81.5 Berens, M.J., Ulrich, B.A., Strehlau, J.H., Hofstetter, T.B., and Arnold, W.A., 2019. Mineral identity, natural organic matter, and repeated contaminant exposures do not affect the carbon and nitrogen isotope fractionation of 2,4-dinitroanisole during abiotic reduction. Environmental Science: Processes and Impacts, 21(1), pp. 51-62. DOI: 10.1039/C8EM00381E
- ^ 82.0 82.1 82.2 82.3 82.4 Strehlau, J.H., Berens, M.J., and Arnold, W.A., 2018. Mineralogy and buffer identity effects on RDX kinetics and intermediates during reaction with natural and synthetic magnetite. Chemosphere, 213, pp. 602–609. DOI: 10.1016/j.chemosphere.2018.09.139
- ^ 83.00 83.01 83.02 83.03 83.04 83.05 83.06 83.07 83.08 83.09 83.10 Cárdenas-Hernandez, P.A., Anderson, K.A., Murillo-Gelvez, J., di Toro, D.M., Allen, H.E., Carbonaro, R.F., and Chiu, P.C., 2020. Reduction of 3-Nitro-1,2,4-Triazol-5-One (NTO) by the Hematite–Aqueous Fe(II) Redox Couple. Environmental Science and Technology, 54(19), pp. 12191–12201. DOI: 10.1021/acs.est.0c03872
- ^ 84.0 84.1 84.2 84.3 84.4 84.5 84.6 84.7 84.8 84.9 Menezes, O., Yu, Y., Root, R.A., Gavazza, S., Chorover, J., Sierra-Alvarez, R., and Field, J.A., 2021. Iron(II) monosulfide (FeS) minerals reductively transform the insensitive munitions compounds 2,4-dinitroanisole (DNAN) and 3-nitro-1,2,4-triazol-5-one (NTO). Chemosphere, 285, p. 131409. DOI: 10.1016/j.chemosphere.2021.131409
- ^ Strathmann, T.J., 2011. Redox Reactivity of Organically Complexed Iron(II) Species with Aquatic Contaminants. Aquatic Redox Chemistry, American Chemical Society,1071(14), pp. 283-313. DOI: 10.1021/bk-2011-1071.ch014
- ^ Linker, B.R., Khatiwada, R., Perdrial, N., Abrell, L., Sierra-Alvarez, R., Field, J.A., and Chorover, J., 2015. Adsorption of novel insensitive munitions compounds at clay mineral and metal oxide surfaces. Environmental Chemistry, 12(1), pp. 74–84. DOI: 10.1071/EN14065
- ^ Jenness, G.R., Giles, S.A., and Shukla, M.K., 2020. Thermodynamic Adsorption States of TNT and DNAN on Corundum and Hematite. The Journal of Physical Chemistry C, 124(25), pp. 13837–13844. DOI: 10.1021/acs.jpcc.0c04512
- ^ Gorski, C.A., and Scherer, M.M., 2011. Fe2+ Sorption at the Fe Oxide-Water Interface: A Revised Conceptual Framework. Aquatic Redox Chemistry, American Chemical Society, 1071(15), pp. 315–343. DOI: 10.1021/bk-2011-1071.ch015
- ^ Gorski, C.A., Aeschbacher, M., Soltermann, D., Voegelin, A., Baeyens, B., Marques Fernandes, M., Hofstetter, T.B., and Sander, M., 2012. Redox Properties of Structural Fe in Clay Minerals. 1. Electrochemical Quantification of Electron-Donating and -Accepting Capacities of Smectites. Environmental Science and Technology, 46(17), pp. 9360–9368. DOI: 10.1021/es3020138
- ^ Gorski, C.A., Klüpfel, L., Voegelin, A., Sander, M., and Hofstetter, T.B., 2012. Redox Properties of Structural Fe in Clay Minerals. 2. Electrochemical and Spectroscopic Characterization of Electron Transfer Irreversibility in Ferruginous Smectite, SWa-1. Environmental Science and Technology, 46(17), pp. 9369–9377. DOI: 10.1021/es302014u
- ^ Gorski, C.A., Klüpfel, L.E., Voegelin, A., Sander, M. and Hofstetter, T.B., 2013. Redox Properties of Structural Fe in Clay Minerals: 3. Relationships between Smectite Redox and Structural Properties. Environmental Science and Technology, 47(23), pp. 13477–13485. DOI: 10.1021/es403824x
- ^ Chen, G., Hofstetter, T.B., and Gorski, C.A., 2020. Role of Carbonate in Thermodynamic Relationships Describing Pollutant Reduction Kinetics by Iron Oxide-Bound Fe2+. Environmental Science and Technology, 54(16), pp. 10109–10117. DOI: 10.1021/acs.est.0c02959
- ^ Bocher, F., Géhin, A., Ruby, C., Ghanbaja, J., Abdelmoula, M., and Génin, J.M.R., 2004. Coprecipitation of Fe(II–III) hydroxycarbonate green rust stabilised by phosphate adsorption. Solid State Sciences, 6(1), pp. 117–124. DOI: 10.1016/j.solidstatesciences.2003.10.004