|
|
| Line 1: |
Line 1: |
| − | Acids are produced during Enhanced Reductive Dechlorination (ERD) which can cause pH to drop, inhibiting treatment. Alkaline materials including hydroxides and carbonates are sometimes added to the aquifer during ERD to maintain a pH of greater than 6, improving bioremediation performance. This article describes a simplified approach and a spreadsheet-based <u>design tool</u> for estimating the total amount of base required to achieve a specified target pH at the end of the treatment period, once all reactions have gone to completion. To prevent overshoot and excessively high pH, users typically add a fraction of the total base required in several increments spread over time.
| + | Landfarming is a well proven ex-situ bioremediation technology that has been successfully used since the 1980s for treating petroleum impacted soils/sediments, drill cuttings, low brine drilling fluids, oily sludges, tank bottoms and pit sludges. The material to be treated is incorporated into surface soil. Naturally occurring microbes in the soil and waste material transform the organic contaminants to carbon dioxide, water and biomass (<ref name= "USEPA1993Bio">U.S. Environmental Protection Agency (USEPA), 1993. Bioremediation using the land treatment concept. US Environmental Protection Agency. Office of Research and Development, Washington, D.C. [[media:USEPA-1993._bio_using_land_treatment.pdf| Report.pdf]]</ref><ref name= "USEPA2003LF">U.S. Environmental Protection Agency (USEPA), 2003. Aerobic Biodegradation of Oily Wastes: A Field Guidance Book for Federal On-scene Coordinators, Version 1.0, October 2003. Region 6 South Central Response and Prevention Branch. [[media:USEPA-2003-Landfarming-OSC-Aerobic_Biodegradation_of_Oily_Wastes.pdf| Report.pdf]]</ref><ref>U.S. Environmental Protection Agency (USEPA), 2017. How to Evaluate Alternative Cleanup Technologies for Underground Storage Tank Sites: A Guide for Corrective Action Plan Reviewers, Chapter V: Landfarming , Land and Emergency Management 5401R, EPA 510-B-17-003. [[media:USEPA-2017._How_to_Evaluate_Alternative_Cleanup_tech_for_UST_Sites.pdf| Report.pdf]]</ref>). Maintaining optimum soil conditions for rapid biodegradation of organic contaminants can help meet cleanup goals within a reasonable timeframe. |
| | | | |
| | <div style="float:right;margin:0 0 2em 2em;">__TOC__</div> | | <div style="float:right;margin:0 0 2em 2em;">__TOC__</div> |
| Line 5: |
Line 5: |
| | | | |
| | '''Related Article(s):''' | | '''Related Article(s):''' |
| − | *[[pH Buffering in Aquifers]] | + | *[[Bioremediation_-_Anaerobic | Anaerobic Bioremediation]] |
| − | *[[Low pH Inhibition of Reductive Dechlorination]]
| |
| − | *[[Bioremediation – Anaerobic]]
| |
| − | *[[Chlorinated Solvents]]
| |
| | | | |
| | | | |
| − | '''CONTRIBUTOR(S):''' [[Dr. Robert Borden, P.E.]] | + | '''CONTRIBUTOR(S):''' [[Roopa Kamath]], [[Sara McMillen]], [[Rene Bernier]], and [[Deyuan Kong]] |
| | | | |
| | | | |
| | '''Key Resource(s):''' | | '''Key Resource(s):''' |
| − | *Spreadsheet-based <u>Design Tool -- Base Addition for ERD</u> | + | *[[media:USEPA-1993._bio_using_land_treatment.pdf| Bioremediation using the land treatment concept.]]<ref name= "USEPA1993Bio"/> |
| − | *[[media:2017-Borden-Post-Remediation_Evaluation_of_EVO_Treatment.pdf| Post-Remediation Evaluation of EVO Treatment: How Can we improve performance]]<ref name = "Borden2017EVO">Borden, R.C., 2017. Post-Remediation Evaluation of EVO Treatment: How Can We Improve Performance. Environmental Security Technology Certification Program, Alexandria, VA. ER-201581[[media:2017-Borden-Post-Remediation_Evaluation_of_EVO_Treatment.pdf| Report.pdf]]</ref> | + | *[[media: USEPA-2003-Landfarming-OSC-Aerobic_Biodegradation_of_Oily_Wastes.pdf| Biotreating E&P wastes: lessons learned from 1992-2003.]]<ref name= "USEPA2003LF"/>. Aerobic Biodegradation of Oily Wastes: A Field Guidance Book for Federal On-scene Coordinators |
| | + | |
| | + | [[File:Kamath1w2 Fig1.png|thumbnail|left|Figure 1: Soils undergoing treatment at a Landfarming Facility]] |
| | | | |
| | ==Introduction== | | ==Introduction== |
| − | Aquifer pH lower than 6 can reduce the efficiency of enhanced reductive dechlorination (ERD) and other in situ remediation processes. This article describes a simplified approach using a spreadsheet-based <u>design tool</u> for estimating the total amount of base required to achieve a specified target pH at the end of the treatment period, once all reactions have gone to completion. This approach requires simplification of several important processes controlling subsurface pH, and is only appropriate for developing initial estimates of the total amount of base required. In practice, aquifer pH should be monitored during ERD and periodically adjusted to maintain conditions for optimal microbial growth and contaminant degradation. Early in the bioremediation process, reactions will not have gone to completion and less base will be required to maintain the target pH. To prevent overshoot and excessively high pH, users typically add a fraction of the total base required in several increments spread over time.
| + | During landfarming, the waste materials are typically placed as a layer on the ground surface with variable thickness. The waste is then tilled and amended with nutrients to enhance biodegradation by naturally occurring bacteria (Figure 1). Fertilizers such as urea and triple superphosphate (TSP) are used to provide nitrogen and phosphate necessary for biodegradation. Reduction in hydrocarbon concentrations can be expected within a span of weeks to months, depending on the initial concentration and composition of hydrocarbons, and whether the soil conditions are optimized for biodegradation. Once cleanup goals have been achieved, the treated material can be i) re-used in construction activity such as berms, landfill cover, backfill, regrading, or for agricultural purposes, ii) disposed at a landfill and/or iii) left in place and revegetated, depending on local regulations or site-specific considerations. |
| − | | |
| − | ==Background Aquifer pH==
| |
| − | In humid areas, rainfall combined with carbonic acid produced in the soil leaches out base cations (Na<sup>+</sup>, K<sup>+</sup>, Ca<sup>2+</sup>, Mg<sup>2+</sup>) gradually acidifying the soil. Figure 1 shows soil pH in the contiguous United States.
| |
| − | | |
| − | [[File:Borden2w2Fig1.png|thumbnail|center|800 px|Figure 1. Soil pH in the contiguous US ([http://www.bonap.org/2008_Soil/pH20110321.png Soil pH]). Alkaline soils (pH>7.4) are shown in blue. Acidic soils (pH<6.1) are shown in brown. ]]
| |
| − | | |
| − | Groundwater pH is influenced by soil pH, but also by other factors. As acidic water infiltrates through the soil profile, some of the acidity may be neutralized by dissolution of soil and aquifer minerals. Silicate minerals including feldspars and micas can hydrolyze over time, consuming acid, and releasing dissolved cations (Na<sup>+</sup>, K<sup>+</sup>, Ca<sup>2+</sup>, Mg<sup>2+</sup>). However, these weathering reactions are slow, and are often not sufficient to prevent pH declines during ERD. Figure 2 shows a cumulative frequency distribution of groundwater pH at a site in eastern North Carolina. Average soil pH at this site is approximately 5. The vadose zone and surficial aquifer at the site are predominantly quartz sand and weathered clays, so weathering processes provide minimal buffering capacity. 90% of the groundwater pH measurements were between 4.5 and 6.0 indicating low soil pH at this site was a reasonable predictor of acidic groundwater. At other sites containing carbonate minerals or relatively young rocks, mineral dissolution often results in greater buffering.
| |
| − | | |
| − | [[File:Borden2w2Fig2.png|thumbnail|left|Figure 2. Cumulative frequency distribution of groundwater pH measurements at site in eastern North Carolina where soil pH is approximately 5. ]]
| |
| − | | |
| − | ==Groundwater Acidity==
| |
| − | Acidity is the amount of base required to neutralize acids present in a water sample. Since various acids can disassociate to different extents, two solutions with the same pH can have different acidities (see <u>pH Buffering in Aquifers</u>). In most cases, the acidity of the background groundwater is the sum of acidity from strong mineral acids (phosphoric, nitric, sulfuric, and hydrochloric acids), organic acids, and dissolved carbon dioxide.
| |
| − | Mineral acidity (also referred to as methyl orange acidity) is determined by titrating a sample with a strong base to pH=3.7 (Standard Method 2310, AWPA 2016). If the initial pH is greater than 3.7, mineral acidity is zero. The spreadsheet-based <u>design tool</u> presented here uses this measurement to account for the base demand of any strong acids already present in the aquifer. Total acidity (commonly referred to as phenolphthalein acidity) should not be used to calculate the amount of base required because the titration endpoint of this parameter is pH 8.3, which will over-estimate the base requirement for optimal ERD.
| |
| − | | |
| − | Under low pH conditions, organic volatile fatty acids (acetic, propionic, etc.) can accumulate and contribute to acidity. However, in background groundwater, organic acid concentrations are generally low. In the design tool, acidity from background organic acids is assumed to be negligible.
| |
| − | | |
| − | In theory, acidity from dissolved carbon dioxide can be measured using a modification of Standard Method 2310<ref name = "APWA2016">American Public Health Association, American Water Works Association and Water Environment Federation (APWA, AWWA, and WEF), 2016. Standard Method 2320 Alkalinity, Standard methods for the examination of water and wastewater. [[media:2016-APWA-Standard_Method_2320_Alkalinity.pdf| Report.pdf]]</ref> in which the sample is titrated to a target pH using a strong base. When using this approach, special precautions should be taken to prevent any pH shift due to degassing of dissolved carbon dioxide from the sample during transport to the laboratory or during analysis of the sample. In the design tool, acidity from background dissolved carbon dioxide is instead calculated from the dissolved inorganic carbon concentration (DIC) and background pH. This eliminates the problem of pH shift due to dissolved carbon dioxide degassing. Commercial laboratories can measure DIC using a modification of the Total Organic Carbon (TOC) procedure.
| |
| − | | |
| − | ==Aquifer Buffering Capacity==
| |
| − | An aquifer’s buffering capacity is the resistance to pH change and is primarily due to two processes: (1) buffering by dissolved and solid carbonates; and (2) surface complexation and/or ion exchange reactions on mineral surfaces. While mineral weathering processes do influence long-term pH changes, weathering reactions are often too slow to prevent pH declines due to HCl and CO<sub>2</sub> production during ERD.
| |
| − | | |
| − | [[File:Borden2w2Fig3.png|thumbnail|right|400 px|Figure 3. Distribution of H<sub>2</sub>CO<sub>3</sub>*, HCO<sub>3</sub><sup>-</sup> and CO<sub>3</sub><sup>2-</sup> as a function of pH. ]]
| |
| | | | |
| − | ''Buffering by Dissolved and Solid Carbonates''
| + | Landfarming is a low-cost technology. Facilities are simple to construct and easy to operate. Standard construction and farming equipment can be used to move soils to the land treatment facility, to amend the soils with fertilizer, to apply water to the soils and to till the soils (e.g. excavator, plow, rotovator, water truck). Figures 2 through 4 show examples of equipment used at a typical landfarming facility. |
| − | In natural aqueous systems, pH buffers are predominantly weak acid anions that easily bind and release hydrogen ions. The most common are the weak acid anions produced by dissolved CO<sub>2</sub> <ref>Langmuir, D., 1997, Aqueous Environmental Geochemistry. Prentice-Hall, Inc. Upper Saddle River, NJ ISBN: 978-0023674129.</ref><ref>Drever, J.I., The Geochemistry of Natural Waters: Surface and Groundwater Environments. Prentice-Hall, Inc., ISBN 0132727900.</ref>. When CO<sub>2</sub> dissolves in water, some CO<sub>2</sub> combines with H<sub>2</sub>O forming carbonic acid (H<sub>2</sub>CO<sub>3</sub>). For convenience, the sum of dissolved CO<sub>2</sub> and H<sub>2</sub>CO<sub>3</sub> is often written as H<sub>2</sub>CO<sub>3</sub>*. H<sub>2</sub>CO<sub>3</sub>*can then disassociate releasing a bicarbonate ion (HCO<sub>3</sub><sup>-</sup>) and one H<sup>+</sup> or a carbonate ion (CO<sub>3</sub><sup>2-</sup>) and two H<sup>+</sup> by the following<ref name= "Stumm1996">Stumm, W. and Morgan, J.J., 1996. Aquatic chemistry; an introduction emphasizing chemical equilibria in natural waters. ISBN-13: 978-0471091738 and ISBN-10: 0471091731</ref>:
| |
| | | | |
| − | <div class="center"><big>H<sub>2</sub>CO<sub>3</sub>* ⇔ H<sup>+</sup> + HCO<sub>3</sub><sup>-</sup> ⇔ 2 H<sup>+</sup> + CO<sub>3</sub><sup>2-</sup> </big> ''Reactions 1 and 2''</div>
| + | [[File:Kamath1w2 Fig2.png|thumbnail|left| Figure 2: Excavator to move soils]] |
| | + | [[File:Kamath1w2 Fig3.png|thumbnail|right| Figure 3: Water truck to ensure optimal moisture content for microbial degradation in soils.]] |
| | + | [[File:Kamath1w2 Fig4.png|thumbnail|right| 600 px| Figure 4: Rotovator and Plows to till soils]] |
| | | | |
| − | Figure 3 shows the relative distribution of these solutes as a function of pH. The reactions are reversible so that (a) an influx of acid will cause the HCO<sub>3</sub><sup>-</sup> and CO<sub>3</sub><sup>2-</sup> ions to protonate, consuming the acid, or (b) an influx of base will cause dissociation (deprotonation) of H<sub>2</sub>CO<sub>3</sub>* and the bicarbonate ion to consume the base. The maximum resistance to pH change (buffering capacity) occurs when the pH is equal to the dissociation constant of either carbonic acid (pH = 6.3 @ 25°C) or of the bicarbonate ion (pH = 10.3 @ 25°C). The buffering capacity of groundwater is measured with an alkalinity titration<ref>U.S. Geological Survey, 2015. National field manual for the collection of water-quality field data, Alkalinity and acid neutralizing capacity. US Geological Survey Techniques of Water-Resources Investigations, book 9, chap. A6., sec. 6.6. [[media:USGS-2015-Natl_Field_Manual.pdf| Report pdf]</ref><ref name= "APWA2016"/>. The units of alkalinity can be given as milliequivalents (meq) per liter, but are often reported as mg CaCO<sub>3</sub>/L (milligrams of CaCO<sub>3</sub> per liter) using the following equation:
| + | Some of the disadvantages of the technology are: |
| | + | *It requires a large land area for treatment. |
| | + | *There may be regulatory limitations on wastes that can be treated by landfarming. For example, U.S. regulations prevent landfarming soil impacted with hazardous wastes such as motor oil, hydraulic oil, and solvents. |
| | + | *It may not be effective for highly impacted soils or soils impacted with severely degraded hydrocarbons (e.g. if soils contain >8% w/w petroleum hydrocarbons after spreading). |
| | + | *Although landfarming is effective for reducing hydrocarbon concentrations, it is not effective for reducing concentrations of other oil field waste components, such as elevated concentrations of metals, salt or wastes containing naturally occurring radioactive materials (NORM). |
| | + | *Concentration reductions >95% or final concentrations <0.1% may not be successfully obtained based on the extent impacted and nature of the hydrocarbons. |
| | + | *Dust and vapor emissions may pose air quality concerns. |
| | | | |
| − | <div class="center">'''Alkalinity''' (mg CaCO<sub>3</sub>/L) '''= Alkalinity''' (meq/L) '''× 50''' (mg CaCO<sub>3</sub>/meq) ''Equation 1''</div>
| + | ==Suitability of Wastes for Landfarming== |
| | + | Landfarming has been successfully used to reduce hydrocarbon concentrations in soils impacted with kerosene, diesel, jet fuel, and crude oil. It has also been successfully used to treat oil-based drilling wastes (drill cutting, low brine drilling), oily sludge, tank bottoms and pit sludges. |
| | | | |
| − | For a system open to a large reservoir of carbon dioxide (e.g. the atmosphere), the buffering capacity (assuming no soluble minerals are present) is a function of the partial pressure of carbon dioxide in the reservoir. Near the water table, groundwater is open to the atmosphere and can release excess dissolved CO<sub>2</sub> gas if its partial pressure is greater than the reservoir’s, essentially stripping some acid out of the groundwater by partially reversing the above reactions.
| + | Wastes may not be suitable for landfarming for a number of reasons: |
| | | | |
| − | In a closed system (e.g. below the water table), the buffering capacity is a function of the total dissolved carbonates (H<sub>2</sub>CO<sub>3</sub>*, HCO<sub>3</sub><sup>-</sup>, and CO<sub>3</sub></sub><sup>-2</sup>). When solid carbonate minerals are present (CaCO<sub>3</sub>, MgCO<sub>3</sub>, CaMg(CO<sub>3</sub>)<sub>2</sub>, FeCO<sub>3</sub>), carbonate mineral dissolution can limit pH declines caused by strong acids (e.g. HCl). For example, in an aquifer containing solid CaCO<sub>3</sub>, the ambient groundwater is saturated with CaCO<sub>3</sub> (s). When HCl is produced by ERD, groundwater pH declines, and dissolved CO<sub>3</sub><sup>2-</sup> also declines as the carbonate ion is protonated by the added H<sup>+</sup> ions causing an increase in HCO<sub>3</sub><sup>-</sup> and/or H<sub>2</sub>CO<sub>3</sub>* (see Figure 3). The groundwater is now under-saturated and CaCO<sub>3</sub> will dissolve, consuming H<sup>+</sup> by the following reaction:
| + | *There may be regulatory or permit limitations. For instance, in the United States, wastes that are not exempted under RCRA <ref>U.S. Environmental Protection Agency (USEPA), 2002. Exemption of Oil and Gas Exploration and Production Wastes from Federal Hazardous Waste Regulations. Office of Solid Waste, EPA530-K-01-004. [[Media:USEPA-2002._Exemption_of_Oil_and_Gas_exploration_and_Production_Wastes....pdf| Report.pdf]]</ref> such as motor oil, hydraulic oil, and solvents cannot be treated by landfarming by law. |
| | + | *Wastes unsuitable by their composition such as solid, non-spreadable paraffins from pig trap scrapings from the maintenance of pipelines, or highly weathered wastes, or asphaltic wastes |
| | + | *Wastes containing contaminants that do not biodegrade: e.g., NORM, metals or salt. |
| | | | |
| − | <div class="center"><big>CaCO<sub>3</sub>(s) + H<sup>+</sup> ⇒ Ca<sup>2+</sup> + HCO<sub>3</sub><sup>-</sup> </big> ''Reaction 3''</div>
| + | ==Biodegradation Potential== |
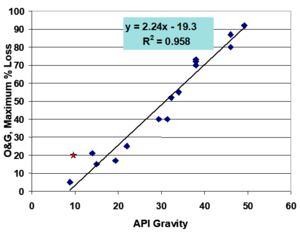
| | + | The initial concentration and composition of hydrocarbons strongly influences the biodegradation potential of a waste. [[File:Kamath1w2 Fig5.png|thumbnail|right| Figure 5. Correlation between API gravity (specific weight of the crude) and the predicted extent of biodegradation as measured by oil and grease (O&G)<ref name= "McMillen2004"/>]] |
| | | | |
| − | [[File:Borden2w2Fig4.png|thumbnail|left|400 px|Figure 4. Buffering capacity of aquifer solids from SA-17 and OU-2 measured in DI water<ref name = "Borden2017EVO"/>. The legend shows depth below ground surface in feet for individual samples. ]]
| + | *Wastes with >5% (w/w) of hydrocarbons may have physical and chemical characteristics that hinder biodegradation. For example, oily wastes tend to repel water, and can be poorly aerated. This can hinder or inhibit biodegradation. |
| − | However, the solubility of carbonate minerals is relatively low, so solid carbonates are much less effective in limiting pH declines due to CO<sub>2</sub> production in the saturated zone. Below the water table, CO<sub>2</sub> may not degas, causing a buildup of HCO<sub>3</sub><sup>-</sup>. Geochemical modeling<ref name="Robinson2009">Robinson, C., Barry, D.A., McCarty, P.L., Gerhard, J.I. and Kouznetsova, I., 2009. pH control for enhanced reductive bioremediation of chlorinated solvent source zones. Science of the Total Environment, 407(16), pp.4560-4573. [http://dx.doi.org/10.1016/j.scitotenv.2009.03.029 doi:10.1016/j.scitotenv.2009.03.029]</ref> indicates that the aquifer can become supersaturated with CaCO<sub>3</sub> during ERD, causing carbonate dissolution to stop. As additional CO<sub>2</sub> is produced, CaCO<sub>3</sub> (s) will precipitate producing H<sup>+</sup> by the following reaction:
| |
| | | | |
| − | <div class="center"><big>H<sub>2</sub>CO<sub>3</sub>* + Ca<sup>2+</sup> + CO<sub>3</sub><sup>2-</sup> ⇒ H<sup>+</sup> + HCO<sub>3</sub><sup>-</sup> + CaCO<sub>3</sub>(s) </big> ''Reaction 4''</div> | + | *The molecular structure and molecular weight of hydrocarbons in the waste may make them either more or less susceptible to attack by microorganisms. Typically, aliphatic hydrocarbons are more readily biodegraded than aromatic hydrocarbons. Structurally more complex molecules such as isoprenoids and steranes are relatively recalcitrant to biodegradation. For crude oils, the API gravity is a reliable indicator of the composition of a crude oil and by extension, a good predictor of the biodegradation potential of a crude oil (see Figure 5)<ref name= "McMillen2004">McMillen, S.J., Smart, R., Bernier, R. and Hoffmann, R.E., 2004, January. Biotreating E&P wastes: lessons learned from 1992-2003. In SPE International Conference on Health, Safety, and Environment in Oil and Gas Exploration and Production. Society of Petroleum Engineers. [https://doi.org/10.2118/86794-ms doi: 10.2118/86794-ms]</ref>. For example, a crude oil with API gravity of 40 (by virtue of its composition) may degrade by 75% within 4 weeks. A crude oil with API gravity of 25 may only degrade by 55%, and a crude oil with API gravity of 10 may not degrade more than 10% within that same timeframe. |
| | | | |
| − | ''Surface Complexation and Ion Exchange Reactions''
| + | This same correlation can be used to determine the maximum initial hydrocarbon concentration that can be treated using conventional landfarming within 4 weeks and/or the maximum endpoint achievable for a given waste material. |
| − | H<sup>+</sup> sorption to Fe and Al oxyhydroxides and clay minerals through surface complexation and ion exchange reactions can have a major impact on pH. H<sup>+</sup> adsorbs strongly to some mineral surfaces, which can accumulate large amounts of H<sup>+</sup> as the solution pH declines and release the H<sup>+</sup> back to solution as the pH rises<ref>Davis, J.A. and Kent, D.B., 1990. Surface complexation modeling in aqueous geochemistry. Reviews in Mineralogy and Geochemistry, 23(1), pp.177-260. [https://doi.org/10.1021/es980312q doi: 10.1021/es980312q ]</ref><ref>Davis, J.A., Coston, J.A., Kent, D.B. and Fuller, C.C., 1998. Application of the surface complexation concept to complex mineral assemblages. Environmental Science & Technology, 32(19), pp.2820-2828. [https://doi.org/10.1021/es980312q doi: 10.1021/es980312q]</ref>. This strong buffer can reduce the pH decline in many systems, but can also greatly increase the amount of base required to increase aquifer pH.
| |
| − | [[File:Borden2w2Fig5.png|thumbnail|right| 400 px|Figure 5. Aquifer buffering capacity (pHBC) from multiple ERD sites<ref name = "Borden2017EVO"/>.]]
| |
| − |
| |
| − | ''Estimating Aquifer Buffering Capacity''
| |
| | | | |
| − | When carbonate minerals are present, geochemical models<ref name="Robinson2009"/> are needed to determine the amount of buffering provided by carbonate mineral dissolution. However, in many naturally low pH aquifers, carbonate minerals are absent and the extent of buffering can be estimated by adding a strong base to the aquifer solids, equilibrating for several days, and measuring the resulting pH. These buffering curves are typically linear in the pH range of 4.5 to 6.5<ref>Magdoff, F.R. and Bartlett, R.J., 1985. Soil pH Buffering Revisited 1. Soil Science Society of America Journal, 49(1), pp.145-148. [https://doi.org/10.2136/sssaj1985.03615995004900010029x doi: 10.2136/sssaj1985.03615995004900010029x]</ref><ref>Liu, M., Kissel, D.E., Cabrera, M.L. and Vendrell, P.F., 2005. Soil lime requirement by direct titration with a single addition of calcium hydroxide. Soil Science Society of America Journal, 69(2), pp.522-530. [https://doi.org/10.2136/sssaj2005.0522 doi:10.2136/sssaj2005.0522]</ref><ref>Aitken, R.L. and Moody, P.W., 1994. The effect of valence and ionic-strength on the measurement of pH buffer capacity. Soil Research, 32(5), pp.975-984. [https://doi.org/10.1071/SR9940975 doi: 10.1071/SR9940975]</ref> recommend carrying out the titrations in a uniform ionic strength solution, most commonly 0.01 M CaCl<sub>2</sub>.
| + | Using analytical methods such as gas chromatography to determine the quantity and composition of organic contaminants in a waste may be useful in determining biodegradation potential. Lighter ends (lower boiling point components that elute earlier in the chromatogram) will typically degrade faster than heavier end components. |
| − | | |
| − | Figure 4 shows the measured final pH versus meq of OH- added per kg of dry aquifer material. Incremental base addition results in a nearly linear increase in pH. The slopes of these curves were estimated by linear regression. pH buffer capacity (pHBC) is calculated as the inverse of the slope and is reported in units of milliequivalents per kilogram (meq/kg) per pH unit.
| |
| − | | |
| − | Figure 5 shows cumulative frequency distributions for pHBC in 6 different contaminated aquifers in the eastern U.S. Within a single unit, pHBC values are reasonably constant, with the middle 50% of measurements varying by a factor of two. However, median pHBC values can vary by a factor of ten between sites and even between sandy versus clayey units on the same site.
| |
| − | | |
| − | ==Acid and Base Production==
| |
| − | | |
| − | Multiple processes can produce or consume acidity during ERD including redox, precipitation, and hydrolysis reactions. Table 1 shows the amount of H<sup>+</sup> released from some important redox reactions.
| |
| − | | |
| − | {| class="wikitable" style="text-align: center;"
| |
| − | |+ colspan="5" | Table 1. Net Acid Production from Important Redox Reactions
| |
| − | |-
| |
| − | ! e<sup>-</sup> Acceptor
| |
| − | ! e<sup>-</sup> Donor
| |
| − | ! Product
| |
| − | ! Reaction
| |
| − | ! H<sup>+</sup> Produced<br/><span style="font-weight: normal; font-size: 85%;">per mole e<sup>-</sup> donor</span>
| |
| − | |-
| |
| − | | PCE || H<sub>2</sub> || TCE || C<sub>2</sub>Cl<sub>4</sub> + H<sub>2</sub> → C<sub>2</sub>H<sub>3</sub>Cl<sub>3</sub> + H<sup>+</sup> + Cl<sup>-</sup> || 1
| |
| − | |-
| |
| − | | TCE || H<sub>2</sub> || ''c''DCE || C<sub>2</sub>HCl<sub>3</sub> + H<sub>2</sub> → C<sub>2</sub>H<sub>2</sub>Cl<sub>2</sub> + H<sup>+</sup> + Cl<sup>-</sup> || 1
| |
| − | |-
| |
| − | | ''c''DCE || H<sub>2</sub> ||VC || C<sub>2</sub>H<sub>2</sub>Cl<sub>2</sub> + H<sub>2</sub> → C<sub>2</sub>H<sub>3</sub>Cl + H<sup>+</sup> + Cl<sup>-</sup> || 1
| |
| − | |-
| |
| − | | VC || H<sub>2</sub> ||Ethene || C<sub>2</sub>H<sub>3</sub>Cl + H<sub>2</sub> → C<sub>2</sub>H<sub>4</sub> + H<sup>+</sup> + Cl<sup>-</sup> || 1
| |
| − | |-
| |
| − | | H<sub>2</sub>O || Acetic Acid ||H<sub>2</sub>, HCO<sub>3</sub><sup>-</sup> || C<sub>2</sub>H<sub>4</sub>O<sub>2</sub> + 4 H<sub>2</sub>O → 2 H<sub>2</sub>CO<sub>3</sub>* + 4 H<sub>2</sub> || 2 (1 - α)
| |
| − | |-
| |
| − | | H<sub>2</sub>O || Lactic Acid ||H<sub>2</sub>, HCO<sub>3</sub><sup>-</sup> || C<sub>3</sub>H<sub>6</sub>O<sub>3</sub> + 3 H<sub>2</sub>O → 3 H<sub>2</sub>CO<sub>3</sub>* + 6 H<sub>2</sub> || 3 (1 - α)
| |
| − | |-
| |
| − | | H<sub>2</sub>O || Glucose ||H<sub>2</sub>, HCO<sub>3</sub><sup>-</sup> || C<sub>6</sub>H<sub>12</sub>O<sub>6</sub> + 12 H<sub>2</sub>O → 6 H<sub>2</sub>CO<sub>3</sub>* + 12 H<sub>2</sub> || 6 (1 - α)
| |
| − | |-
| |
| − | | H<sub>2</sub>O || Soybean Oil ||H<sub>2</sub>, HCO<sub>3</sub><sup>-</sup> || C<sub>56</sub>H<sub>100</sub>O<sub>6</sub> + 162 H<sub>2</sub>O → 56 H<sub>2</sub>CO<sub>3</sub>* + 156 H<sub>2</sub> || 56 (1 - α)
| |
| − | |-
| |
| − | | Oxygen || H<sub>2</sub> ||H<sub>2</sub>O || O<sub>2</sub> + 2 H<sub>2</sub> → 2 H<sub>2</sub>O || 0
| |
| − | |-
| |
| − | | Nitrate || H<sub>2</sub> ||N<sub>2</sub>, OH<sup>-</sup> || NO<sub>3</sub><sup>-</sup> + 2 ½ H<sub>2</sub> → 2 H<sub>2</sub>O + ½ N<sub>2</sub> + OH<sup>-</sup>|| -1
| |
| − | |-
| |
| − | | Goethite || H<sub>2</sub> ||Fe<sup>2+</sup>, OH<sup>-</sup> || FeO(OH) + ½ H<sub>2</sub> → Fe<sup>2+</sup> + 2 OH<sup>-</sup>|| -2
| |
| − | |-
| |
| − | | Sulfate || H<sub>2</sub> || HS<sup>-</sup> || SO<sub>4</sub><sup>2-</sup> + 4 H<sub>2</sub> + Fe<sup>2+</sup> → FeS + 4 H<sub>2</sub>O || 0
| |
| − | |-
| |
| − | | H<sub>2</sub>CO<sub>3</sub>* || H<sub>2</sub> || CH<sub>4</sub> || H<sub>2</sub>CO<sub>3</sub>* + 4 H<sub>2</sub> → CH<sub>4</sub> + 2 H<sub>2</sub>O|| α - 1
| |
| − | |}
| |
| | | | |
| − | During reductive dechlorination of PCE (C<sub>2</sub>Cl<sub>4</sub>) to TCE (C<sub>2</sub>HCl<sub>3</sub>), a chlorine atom (Cl) is replaced with hydrogen (H<sub>2</sub>) releasing one proton (H<sup>+</sup>) and one chloride (Cl<sup>-</sup>).
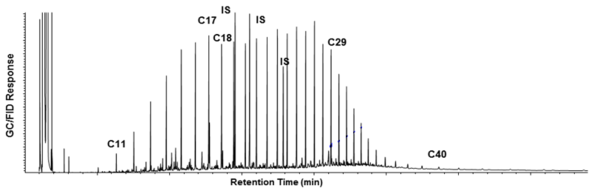
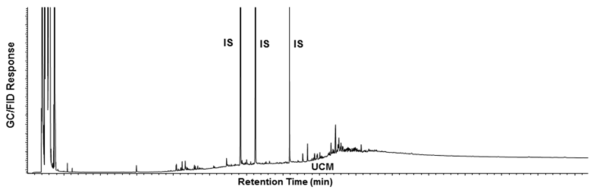
| + | *The extent of weathering (volatilization and biodegradation) may influence the rate and extent of biodegradation. Fresh crude oils with API gravity >30 are generally readily biodegradable; however, after extensive weathering as might be encountered at an old spill site, the oil may not be very biodegradable as most of the more volatile and biodegradable fractions of hydrocarbon have already disappeared. It is recommended that feasibility evaluations include hydrocarbon analysis of wastes using gas chromatography (GC) to assess the extent of weathering. (Figures 6a and 6b) |
| | | | |
| − | <div class="center"><big>C<sub>2</sub>Cl<sub>4</sub> + H<sub>2</sub> ⇒ C<sub>2</sub>HCl<sub>3</sub> + H<sup>+</sup> + Cl<sup>-</sup> </big> ''Reaction 5''</div>
| + | [[File:Kamath1w2 Fig6a.png|thumbnail|right|600 px| Figure 6a: Chromatogram of fresh crude oil]] |
| | | | |
| − | As TCE is further dechlorinated to ''c''DCE, VC and ethene, an additional proton is released for each chlorine removed. As a result, ERD can release large amounts of acid. For example, complete reduction of one kilogram of PCE to ethene produces 0.9 kilograms of HCl.
| + | [[File:Kamath1w2 Fig6b.png|thumbnail|right|600 px|Figure 6b: Chromatogram of weathered crude oil]] |
| | | | |
| − | Organic substrates added as electron donors ferment in oxygen-poor environments, releasing H<sub>2</sub> and acetic acid. Acetic acid rarely accumulates during ERD, because it can be used by various cohorts of organisms for reduction of PCE and TCE, for reduction of background electron acceptors, or for fermentation to methane (CH<sub>4</sub>). Consumption of acetic acid by all of these processes produces H<sub>2</sub>CO<sub>3</sub>*. The amount of H<sub>2</sub>CO<sub>3</sub>* produced during fermentation of different substrates can be estimated with the following formula:
| + | *Light refined products, such as diesel and jet fuels, are highly biodegradable, similar to crude oils with API gravity >40. Heavy refined products like Bunker C or heavy fuel oils will biodegrade at rates similar to low API-gravity crude oils. |
| | | | |
| − | <div class="center"><big>C<sub>''θ''</sub>H<sub>''β''</sub>O<sub>''γ''</sub> + (3''θ'' - ''γ'')H<sub>2</sub>O ⇒ ''θ''H<sub>2</sub>CO<sub>3</sub>* + (2''θ'' + ''β''/2 - ''γ'')H<sub>2</sub> </big> ''Reaction 6''</div>
| + | ==Facility Design and Operation== |
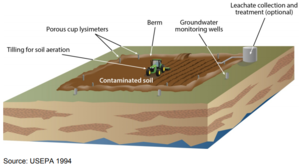
| | + | Landfarming facilities are designed to prevent impacts to groundwater and surface water. The facilities typically include an impermeable or low permeability liner for the treatment pad to prevent leaching of chemical constituents from the treatment waste to the groundwater, and berms/dikes to prevent storm water runoff. (See Figure 7.) |
| | + | [[File:Kamath1w2 Fig7.PNG|thumbnail| center| Figure 7: Typical Landfarming Operation]] |
| | + | Successful landfarm operation requires maintaining optimum conditions in the soil/waste materials (moisture, oxygen, pH and nutrient levels) to enhance biodegradation of petroleum hydrocarbons (see Table 1). Before beginning landfarm operations, the waste and the soil at the landfarm site should be characterized to establish the soil baseline characteristics and the need for soil additives such as agricultural lime for pH correction. |
| | | | |
| − | {| | + | {| class="wikitable" style="text-align: left;" |
| | + | |+ colspan="2" | Table 1. Optimum Conditions for Landfarming |
| | |- | | |- |
| − | |where:
| + | ! Parameter |
| − | |-
| + | ! Optimum Condition |
| − | | ''θ'' || is the number of carbon atoms per mole of substrate
| |
| − | |-
| |
| − | | ''β'' || is the number of hydrogen atoms per mole of substrate
| |
| − | |-
| |
| − | | ''γ'' || is the number of oxygen atoms per mole of substrate
| |
| − | |}
| |
| − | <br/>
| |
| − | | |
| − | [[File:Borden2w2Fig6.png|thumbnail|right|Figure 6. Fraction of H<sub>2</sub>CO<sub>3</sub>* not ionized (α) versus pH. ]]
| |
| − | As shown in Figure 3, CO<sub>3</sub><sup>2-</sup> is only present in significant concentrations at pH > 8. For pH ranges relevant for ERD (5>pH>8), H<sup>+</sup> release from H<sub>2</sub>CO<sub>3</sub>* can be represented by the following reaction<ref name= "Stumm1996"/>.
| |
| − | | |
| − | <div class="center"><big>H<sub>2</sub>CO<sub>3</sub>* ⇒ ''α'' H<sub>2</sub>CO<sub>3</sub>* + (1 - ''α'') H<sup>+</sup> + (1 - ''α'') HCO<sub>3</sub><sup>-</sup> </big> ''Reaction 7''</div>
| |
| − | | |
| − | where α is the fraction of H<sub>2</sub>CO<sub>3</sub>* that does NOT ionize at the target pH with α = (1+ 10<sup>-6.352</sup> / [H<sup>+</sup>])<sup>-1</sup>. Figure 6 shows the variation in α as a function of pH. At pH 6, α = 0.69, so 0.31 moles of H<sup>+</sup> are released for every mole of CO<sub>2</sub> produced. However at pH 7, α = 0.18, so 0.82 moles of H<sup>+</sup> are released for every mole of CO<sub>2</sub> produced.
| |
| − | | |
| − | Reduction of some electron acceptors produces OH<sup>-</sup>, counter-acting acid produced from dechlorination and CO<sub>2</sub> production. Representative reactions for reduction of nitrate (NO<sub>3</sub><sup>-</sup>), iron oxides, sulfate (SO<sub>4</sub><sup>2-</sup>) and bicarbonate (HCO<sub>3</sub><sup>-</sup>) are included in Table 1. Goethite [FeO(OH)] is used as a typical iron oxide for these calculations.
| |
| − | | |
| − | ==Base Addition==
| |
| − | A variety of different bases have been used to raise the aquifer pH to stimulate ERD including soluble and solid carbonates, soluble and solid hydroxides, phosphates and silicate minerals. The carbonates and hydroxides are used most commonly because of their relatively low cost and easy availability. Table 2 summarizes the physical properties of common basic salts used to raise pH.
| |
| − | | |
| − | {| class="wikitable" style="text-align: center;"
| |
| − | |+ colspan="7" | Table 2. Physical Properties of Common Basic Salts used for pH Control
| |
| | |- | | |- |
| − | ! Base
| + | | Initial HC Concentration || 1% - 5% by weight in dry soil |
| − | ! Formula
| |
| − | ! Molecular<br/>Weight<br/><span style="font-weight: normal; font-size: 85%;">(g/mole)</span>
| |
| − | ! OH<sup>-</sup><br/><span style="font-weight: normal; font-size: 85%;">(eq/mole)</span>
| |
| − | ! OH<sup>-</sup><br/><span style="font-weight: normal; font-size: 85%;">(eq/kg Base)</span>
| |
| − | ! Solubility<br/><span style="font-weight: normal; font-size: 85%;">(g/L)</span>
| |
| − | ! Saturated<br/>solution pH
| |
| | |- | | |- |
| − | | Caustic Soda || NaOH || 40.0 || 1 || 25.0 || 1,100 || >14 | + | | pH || 6 – 8.5. Agricultural lime can be added to increase soil pH. |
| − | |-
| |
| − | | Caustic Potash || KOH || 56.1 || 1 || 17.8 || 1,200 || >14
| |
| | |- | | |- |
| − | | Soda Ash || Na<sub>2</sub>CO<sub>3</sub> || 106 || 1 + α || 11.2 || 300 || ~11.7 | + | | Aeration/Tilling || Initially and every 2 – 4 weeks. |
| | |- | | |- |
| − | | Baking Soda || NaHCO<sub>3</sub> || 84 || α || 2.2 || 78 || ~8.3 | + | | Moisture Content || 60% – 80% of water holding capacity (field capacity). |
| | |- | | |- |
| − | | Hydrated Lime || Ca(OH)<sub>2</sub> || 74.1 || 2 || 27.0 || 1.85 || ~12.4 | + | | Salt Content || Electrical conductance of leachate <30 mS/cm (mhos/cm). Preferably <4 mS/cm for maximum reuse potential after cleanup. Ocean water has an electrical conductance of ~50 mS/cm. |
| | |- | | |- |
| − | | Magnesium<br/>Hydroxide ||Mg(OH)<sub>2</sub> || 58.3 || 2 || 34.3 || <0.01 || ~10.3 | + | | Nutrients || |
| | + | * Total Nitrogen: 250 – 500 ppm. Action Level: 50 ppm. Nitrogen source should be added if nutrient concentration falls below action level (e.g. urea). |
| | + | * Available phosphate: 125 – 250 ppm. Action Level: 25 ppm. Phosphate source should be added if nutrient concentration falls below action level (e.g. triple superphosphate). |
| | |} | | |} |
| − | <br/>
| |
| − | NaOH and KOH provide a large number of OH- equivalents (eq) per kg and are very soluble so only small volumes of base are required to raise the aquifer pH. However, concentrated solutions of NaOH and KOH have pH > 14 which is inhibitory to bacteria, would expose workers to safety hazards, and can partially dissolve aluminosilicates.
| |
| | | | |
| − | As described above, solid calcium carbonate (CaCO<sub>3</sub>) is generally not effective in raising pH during ERD because of its low aqueous solubility. Na<sub>2</sub>CO<sub>3</sub> and NaHCO<sub>3</sub> are much more soluble and can be effective in raising aquifer pH. The amount of H<sup>+</sup> consumed per mole varies as a function of pH between pH 5 and 8<ref name= "Stumm1996"/>. For closed conditions (below water table where CO<sub>2</sub> cannot degas) and pH < 8, NaHCO<sub>3</sub> and Na<sub>2</sub>CO<sub>3</sub> disassociate to H<sub>2</sub>CO<sub>3</sub>* and HCO<sub>3</sub><sup>-</sup>, consuming H<sup>+</sup> by the following reactions.
| |
| − |
| |
| − | <div class="center"><big>NaHCO<sub>3</sub> + ''α'' H<sup>+</sup> ⇒ Na<sup>+</sup> + ''α '' H<sub>2</sub>CO<sub>3</sub>* + (1 – ''α'') HCO<sub>3</sub><sup>-</sup> </big> ''Reaction 8''</div>
| |
| − |
| |
| − | <div class="center"><big>Na<sub>2</sub>CO<sub>3</sub> + (1 + ''α'') H<sup>+</sup> ⇒ 2 Na<sup>+</sup> + ''α'' H<sub>2</sub>CO<sub>3</sub>* + (1 – ''α'') HCO<sub>3</sub><sup>-</sup> </big> ''Reaction 9''</div>
| |
| − |
| |
| − | At pH = 6, α = 0.69 so 0.69 moles of H<sup>+</sup> are consumed per mole of NaHCO<sub>3</sub> and 1.69 moles of H<sup>+</sup> consumed per mole of Na<sub>2</sub>CO<sub>3</sub> (Figure 6). However at pH = 7, α = 0.18 so only 0.18 moles of H<sup>+</sup> are consumed per mole of NaHCO<sub>3</sub> and 1.18 moles of H<sup>+</sup> consumed per mole of Na2CO<sub>3</sub>. As a result, bicarbonates and carbonates are relatively effective at raising the pH to 6. However, these materials provide less alkalinity per unit mass at pH =7, increasing the amount of material required.
| |
| − |
| |
| − | Ca(OH) <sub>2</sub> and Mg(OH) <sub>2</sub> provide large amounts of OH- per kg. However, these materials have a low aqueous solubility, making them more difficult to distribute in the subsurface.<ref>Borden, R.C., Lai, Y.S., Overmeyer, J., Yuncu, B. and Allen, J.P., 2016. In situ pH adjustment with colloidal Mg(OH)<sub>2</sub>. Environmental Engineer and Scientist: Applied Research and Practice, accepted for publication, Vol 17, pp.28-33.</ref> describe the use of a colloidal form of Mg(OH) <sub>2</sub> with improved transport properties.
| |
| − |
| |
| − | ==Design Tool==
| |
| − | An MS Excel based <u>design tool</u> was developed to aid in estimating the amount of base required to achieve a specified target pH at the end of the treatment period, once all reactions have gone to completion<ref name = "Borden2017EVO"/>. The general approach and calculations were presented in the previous sections. The design tool is specifically focused on pH adjustment for ERD and calculation procedures include the following assumptions.
| |
| − |
| |
| − | #The target pH is between 5 and 8.
| |
| − | #The carbonate system is the primary aqueous pH buffer.
| |
| − | #Organic acids including volatile fatty acids (acetic, propionic, etc.) are consumed and do not accumulate.
| |
| − | #Any H<sub>2</sub> or acetate produced by substrate fermentation that is not consumed through reduction of chlorinated solvents and background electron acceptors (O<sub>2</sub>, NO<sub>3</sub>, iron oxides, and SO<sub>4</sub>) is consumed by methanogenesis.
| |
| − | #All processes and reactions have gone to completion. The calculated total base requirement is for the end of the treatment period once all acids (HCl and H<sub>2</sub>CO<sub>3</sub>) have been produced.
| |
| − |
| |
| − | In general, the most important factors controlling base requirement are: a) initial and target pH; b) amount of substrate consumed; c) aquifer buffering capacity (pHBC); and d) amount of iron oxides reduced. To calculate base requirement, design tool users must enter the following information.
| |
| − |
| |
| − | #Treatment zone dimensions and design period for this phase of remediation.
| |
| − | #Site characteristics including average hydraulic conductivity (K), porosity, hydraulic gradient, contaminant concentrations in aquifer material and groundwater, and amount of electron acceptors to be produced (i.e. CH<sub>4</sub>) or consumed (O<sub>2</sub>, NO<sub>3</sub>, Fe, SO<sub>4</sub>).
| |
| − | #Background pH, total inorganic carbon, mineral acidity, and pH buffering capacity (pHBC). A database of pHBC measurements is provided to aid users in selecting design values when laboratory measurements are not available.
| |
| − | #Mass of organic substrate and base to be injected.
| |
| − | #When vegetable oil is used as a substrate, the fraction of injected oil that is consumed during the design period.
| |
| − | #Target pH
| |
| − |
| |
| − | Users are reminded that the design tool calculates the total amount of base required '''once all reactions go to completion'''. Early in the bioremediation process, reactions will not have gone to completion and less base will be required to maintain the target pH. To prevent overshoot and excessively high pH, users typically add a fraction of the total base required in several increments spread over time.
| |
| − |
| |
| − | [[File:Borden2w2Fig7.png|400 px|thumbnail|left|Figure 7. Required base addition for PRB for different target pH values.]]
| |
| − |
| |
| − | ==Case Study==
| |
| − | The design tool was used to estimate the amount of base required to stimulate ERD in a 435 ft long emulsified vegetable oil (EVO) biobarrier at Operable Unit 2 (OU2) on the former Naval Training Center (NTC) in Orlando, FL<ref name = "Borden2017EVO"/>. The biobarrier consists of two rows of injection wells spaced approximately 30 ft on center which were treated with a total of 20,224 lb of vegetable oil. The vertical treatment thickness was 10 ft, influent groundwater pH ~5, and pHBC = 8 meq/kg/pH. To provide guidance on amounts of base required, the design tool was used to estimate the amount of NaOH, Na<sub>2</sub>CO<sub>3</sub>, NaHCO<sub>3</sub>, or Mg(OH) <sub>2</sub> required to achieve different pH values at the end of the five year treatment period. Results presented in Figure 7 indicate that the amount of base required is very sensitive to the target pH. For target pH values < 5.4, little or no base is required. Increasing the target pH to 6 or 7, requires progressively greater amounts of base because of the soil acidity and carbonic acid released from substrate fermentation.
| |
| − |
| |
| − | To achieve a pH of approximately 7, 10,200 lb of NaOH or 7,400 lb of Mg(OH) <sub>2</sub> are required, which is equivalent to 50% or 37% respectively (by weight) of the vegetable oil injected. Multiple NaOH injections would be required since a single injection would result in an excessively high initial pH and the base would migrate out of the treatment zone over time with flowing groundwater. The total mass of Mg(OH) <sub>2</sub> required is less than NaOH, since two moles of OH- are released per mole of Mg(OH) <sub>2.</sub> However, Mg(OH) <sub>2</sub> has a very low aqueous solubility, so the material must be injected in a colloidal form. Since the material is a solid and would not migrate downgradient, 100% of the required Mg(OH) <sub>2</sub> could be injected simultaneously with the EVO. The pH of a pure slurry of Mg(OH)<sub>2</sub> is relatively high (~10.3). However once injected, CO<sub>3</sub><sup>2-</sup> precipitates on the surface of the Mg(OH) <sub>2</sub> particles forming a MgCO<sub>3</sub> coating that maintains the aquifer pH between 7 and 8<ref>Hiortdahl, K.M. and Borden, R.C., 2013. Enhanced reductive dechlorination of tetrachloroethene dense nonaqueous phase liquid with EVO and Mg (OH)2. Environmental science & technology, 48(1), pp.624-631. [https://doi.org/10.1021/es4042379 doi: 10.1021/es4042379]</ref>. In general, it is not practical to raise the pH to near 7 with NaHCO<sub>3</sub> due to the small amount of H<sup>+</sup> consumed by this material at near neutral pH. Na<sub>2</sub>CO<sub>3</sub> could be effective, but would require multiple injections due to the high pH (~11.7) and downgradient migration with groundwater flow.
| |
| − |
| |
| − | ==Summary==
| |
| − | Alkaline materials are commonly added during ERD to maintain an aquifer pH of greater than 6. A simplified approach and spreadsheet-based design tool are presented for estimating the total amount of base required to achieve a specified target pH at the end of the treatment period, once all reactions have gone to completion. To prevent overshoot and excessively high pH, users typically add a fraction of the total base required in several increments spread over time. Users should be aware that results are very sensitive to variations in the target pH since the amount of H<sup>+</sup> released from carbon dioxide and consumed by NaHCO<sub>3</sub> and Na<sub>2</sub>CO<sub>3</sub> is a function of pH.
| |
| | | | |
| | ==References== | | ==References== |
| Line 219: |
Line 93: |
| | | | |
| | ==See Also== | | ==See Also== |
| − |
| |
| − | *[https://infoscience.epfl.ch/record/124950 BUCHLORAC: Program for predicting the bicarbonate requirement for anaerobic bioremediation of chlorinated solvents]
| |