Difference between revisions of "User:Debra Tabron/sandbox"
Debra Tabron (talk | contribs) (Replaced content with " <div style="float:right;margin:0 0 2em 2em;">__TOC__</div> '''Related Article(s):''' '''CONTRIBUTOR(S):''' Paul Hatzinger '''Key Resource(s)''': ==Introduction...") (Tag: Replaced) |
Debra Tabron (talk | contribs) |
||
| Line 1: | Line 1: | ||
| − | + | Nonionic and nonpolar organic contaminants including chlorinated solvents and petroleum hydrocarbons can become associated with solid phase organic matter in the soil, sediment, and rock, slowing contaminant migration in groundwater and reducing the efficacy of many remedial technologies. When amorphous organic matter is the primary sorbent, sorption is linear and distribution coefficients can be estimated from empirical correlations with contaminant properties (aqueous solubility or octanol-water partition coefficient) and site specific measurements of solid phase organic carbon. However, when thermally altered carbonaceous materials (TACMs) are present, sorption may be non-linear and the empirical correlations may substantially underestimate sorption at low contaminant concentrations. | |
<div style="float:right;margin:0 0 2em 2em;">__TOC__</div> | <div style="float:right;margin:0 0 2em 2em;">__TOC__</div> | ||
'''Related Article(s):''' | '''Related Article(s):''' | ||
| + | *[[Chlorinated Solvents]] | ||
| + | *[[Petroleum Hydrocarbons]] | ||
| − | '''CONTRIBUTOR(S):''' [[ | + | '''CONTRIBUTOR(S):''' [[Richelle Allen-King]] |
'''Key Resource(s)''': | '''Key Resource(s)''': | ||
| + | *[[media:1990-Piwoni-Basic_concepts_sorption_haz_site_EPA_540-4-90-053.pdf| Basic Concepts of Contaminant Sorption at Hazardous Waste Sites]]<ref>Piwoni, M.D. and Keeley, J.W., 1990. Basic concepts of contaminant sorption at hazardous waste sites. Ground-Water Issue; EPA-540/4-90/053. [[media:1990-Piwoni-Basic_concepts_sorption_haz_site_EPA_540-4-90-053.pdf| Report.pdf]]</ref> | ||
| + | |||
| + | ==Introduction== | ||
| + | Sorption is a non-mechanistic term that describes uptake of a contaminant by a solid phase sorbent (soil, sediment, rock or an engineered material such as [https://en.wikipedia.org/wiki/Activated_carbon activated carbon]). This article focuses on sorption of nonionic and nonpolar organic contaminants and their associations with subsurface materials. For these contaminants, sorption is almost exclusively associated with organic matter in the solids and is a reversible process. Sorption of ionic and polar organic contaminants is not described in this article. | ||
| + | |||
| + | In this article, the term ‘sorption’ includes all processes that cause the dissolved organic compound (sorbate) to become associated with the solid phase (sorbent). For nonpolar organic compounds, sorption occurs by either adsorption or absorption. [https://en.wikipedia.org/wiki/Absorption_(chemistry) Absorption] is a volume-based process whereby the sorbate diffuses into the organic matter, analogous to the way that organic contaminants partition between immiscible oil and aqueous phases. In contrast, [https://en.wikipedia.org/wiki/Adsorption adsorption] describes the interaction between the contaminant and the sorbent surfaces. | ||
| + | |||
| + | ==Measuring Sorption== | ||
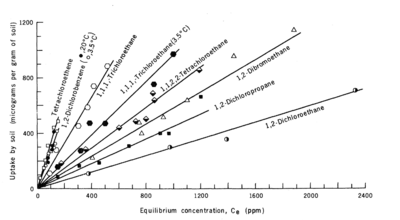
| + | Sorption can be measured in the laboratory by placing a known mass of the contaminant, water, and uncontaminated sediment in a batch reactor (Fig. 1a). The system is mixed for a specific duration (contact time) and then the aqueous and sorbed contaminant concentrations are determined. This process is repeated, adding a range of different initial contaminant masses to achieve a range of aqueous contaminant concentrations. When the experiment is run at constant temperature until the concentrations in the solid and liquid phases approach equilibrium, the resulting data plot is termed an isotherm. Figure 1b shows linear sorption isotherms for eight compounds measured in one soil. | ||
| + | |||
| + | [[File:Allen-King1w2 Fig1a.png|right|thumb| 400 px|Figure 1a. Batch reactor experiments Part (a) to generate points on a sorption isotherm (b). Part (a) to show two samples prepared in duplicate with soil-free control vials and vials to verify the contaminant mass added to the systems<ref name= "Chiou1983">Chiou, C.T., Porter, P.E. and Schmedding, D.W., 1983. Partition equilibriums of nonionic organic compounds between soil organic matter and water. Environmental Science & Technology, 17(4), pp.227-231. [http://dx.doi.org/10.1021/es00110a009 doi: 10.1021/es00110a009]</ref>.]][[File:Allen-King1w2 Fig1b.png|thumb|400 px|Figure 1b. Batch reactor experiments Part (b) to show linear isotherms for eight compounds in one soil<ref name= "Chiou1983"/>. ]] | ||
| + | |||
| + | The time required to reach equilibrium can vary depending on the contaminant and sorbent. In studies with aquifer material from Canadian Forces Base Borden, tetrachloroethene (PCE) sorption reached equilibrium within 2 - 4 weeks, but tetrachlorobenzene did not reach equilibrium after 100 days. Powdered (pulverized) samples equilibrated more quickly indicating mass transfer within the grains was limiting<ref>Ball, W.P. and Roberts, P.V., 1991. Long-term sorption of halogenated organic chemicals by aquifer material. 2. Intraparticle diffusion. Environmental Science & Technology, 25(7), pp.1237-1249. [http://dx.doi.org/10.1021/es00019a003 doi:10.1021/es00019a003]</ref>. | ||
| + | In some cases, the experimental data show an approximately linear relationship between aqueous and sorbed concentration, and the experimental results can be summarized in the form of a partition or distribution coefficient, ''K<sub>d</sub>'': </br> | ||
| + | |||
| + | [[File:Allen-King1w2 Eq1.png|300 px]] | ||
| + | |||
| + | where ''C<sub>S</sub>'' is the solid (sorbed) concentration and ''C<sub>w<sub>'' is the aqueous (dissolved) concentration. By convention, ''C<sub>s</sub>'' has units of µg/g and ''C<sub>s</sub>'' has units of µg/mL, so ''K<sub>d</sub>'' has units of mL/g. | ||
| + | |||
| + | When the relationship between ''C<sub>s</sub>'' and ''C<sub>w</sub>'' is non-linear, the experimental data are commonly represented by the Freundlich isotherm equation:</br> | ||
| + | [[File:Allen-King1w2 Eq2.png|300px]] | ||
| + | |||
| + | where K''<sub>F</sub>'' and ''n'' are empirically estimated coefficients specific to the sorbate, sorbent and equation used. | ||
| + | |||
| + | In most cases, ''absorption'' processes follow a linear isotherm. However, ''adsorption'' processes often follow a nonlinear relationship between the sorbed and dissolved contaminant concentrations. When both absorption and adsorption occur, a non-linear relationship is used to represent the sorption results. | ||
| + | |||
| + | ==Impact on Groundwater Transport== | ||
| + | Sorption reduces the amount of contaminant in the aqueous phase, slowing contaminant migration. Sorbed contaminants are also less available for biological or chemical degradation reactions, which will reduce the effectiveness of many remedial technologies. | ||
| + | |||
| + | The average rate that a contaminant migrates in groundwater is reduced because the sorbed portion of the contaminant does not migrate. The impact of sorption on the average contaminant transport velocity can be estimated using a Retardation Factor (''R''): | ||
| − | |||
Revision as of 17:37, 20 February 2019
Nonionic and nonpolar organic contaminants including chlorinated solvents and petroleum hydrocarbons can become associated with solid phase organic matter in the soil, sediment, and rock, slowing contaminant migration in groundwater and reducing the efficacy of many remedial technologies. When amorphous organic matter is the primary sorbent, sorption is linear and distribution coefficients can be estimated from empirical correlations with contaminant properties (aqueous solubility or octanol-water partition coefficient) and site specific measurements of solid phase organic carbon. However, when thermally altered carbonaceous materials (TACMs) are present, sorption may be non-linear and the empirical correlations may substantially underestimate sorption at low contaminant concentrations.
Related Article(s):
CONTRIBUTOR(S): Richelle Allen-King
Key Resource(s):
Introduction
Sorption is a non-mechanistic term that describes uptake of a contaminant by a solid phase sorbent (soil, sediment, rock or an engineered material such as activated carbon). This article focuses on sorption of nonionic and nonpolar organic contaminants and their associations with subsurface materials. For these contaminants, sorption is almost exclusively associated with organic matter in the solids and is a reversible process. Sorption of ionic and polar organic contaminants is not described in this article.
In this article, the term ‘sorption’ includes all processes that cause the dissolved organic compound (sorbate) to become associated with the solid phase (sorbent). For nonpolar organic compounds, sorption occurs by either adsorption or absorption. Absorption is a volume-based process whereby the sorbate diffuses into the organic matter, analogous to the way that organic contaminants partition between immiscible oil and aqueous phases. In contrast, adsorption describes the interaction between the contaminant and the sorbent surfaces.
Measuring Sorption
Sorption can be measured in the laboratory by placing a known mass of the contaminant, water, and uncontaminated sediment in a batch reactor (Fig. 1a). The system is mixed for a specific duration (contact time) and then the aqueous and sorbed contaminant concentrations are determined. This process is repeated, adding a range of different initial contaminant masses to achieve a range of aqueous contaminant concentrations. When the experiment is run at constant temperature until the concentrations in the solid and liquid phases approach equilibrium, the resulting data plot is termed an isotherm. Figure 1b shows linear sorption isotherms for eight compounds measured in one soil.

The time required to reach equilibrium can vary depending on the contaminant and sorbent. In studies with aquifer material from Canadian Forces Base Borden, tetrachloroethene (PCE) sorption reached equilibrium within 2 - 4 weeks, but tetrachlorobenzene did not reach equilibrium after 100 days. Powdered (pulverized) samples equilibrated more quickly indicating mass transfer within the grains was limiting[3].
In some cases, the experimental data show an approximately linear relationship between aqueous and sorbed concentration, and the experimental results can be summarized in the form of a partition or distribution coefficient, Kd:
where CS is the solid (sorbed) concentration and Cw is the aqueous (dissolved) concentration. By convention, Cs has units of µg/g and Cs has units of µg/mL, so Kd has units of mL/g.
When the relationship between Cs and Cw is non-linear, the experimental data are commonly represented by the Freundlich isotherm equation:
![]()
where KF and n are empirically estimated coefficients specific to the sorbate, sorbent and equation used.
In most cases, absorption processes follow a linear isotherm. However, adsorption processes often follow a nonlinear relationship between the sorbed and dissolved contaminant concentrations. When both absorption and adsorption occur, a non-linear relationship is used to represent the sorption results.
Impact on Groundwater Transport
Sorption reduces the amount of contaminant in the aqueous phase, slowing contaminant migration. Sorbed contaminants are also less available for biological or chemical degradation reactions, which will reduce the effectiveness of many remedial technologies.
The average rate that a contaminant migrates in groundwater is reduced because the sorbed portion of the contaminant does not migrate. The impact of sorption on the average contaminant transport velocity can be estimated using a Retardation Factor (R):
References
- ^ Piwoni, M.D. and Keeley, J.W., 1990. Basic concepts of contaminant sorption at hazardous waste sites. Ground-Water Issue; EPA-540/4-90/053. Report.pdf
- ^ 2.0 2.1 Chiou, C.T., Porter, P.E. and Schmedding, D.W., 1983. Partition equilibriums of nonionic organic compounds between soil organic matter and water. Environmental Science & Technology, 17(4), pp.227-231. doi: 10.1021/es00110a009
- ^ Ball, W.P. and Roberts, P.V., 1991. Long-term sorption of halogenated organic chemicals by aquifer material. 2. Intraparticle diffusion. Environmental Science & Technology, 25(7), pp.1237-1249. doi:10.1021/es00019a003