Difference between revisions of "User:Debra Tabron/sandbox"
Debra Tabron (talk | contribs) (Replaced content with " <div style="float:right;margin:0 0 2em 2em;">__TOC__</div> '''Related Article(s)''': *Climate Change Resiliency (Coming soon) *Climate Change Prediction Datasets...") (Tag: Replaced) |
Debra Tabron (talk | contribs) |
||
| Line 1: | Line 1: | ||
| − | + | Many contaminated sites use active remedies such as pump-and-treat or ''in situ'' remediation to clean up impacted groundwater. Natural attenuation processes such as natural degradation or hydrodynamic dispersion also contribute to the cleanup. As remediation progresses, a point is often reached when the time required to reach the remedial objectives using the active remedy is roughly the same as the time required if the active remedy is shut down, and the continuing remediation of the site is provided by natural attenuation processes alone. From that point forward, the extra effort and expense of the active remedy provides no benefit over natural attenuation, and it may be appropriate to transition the site to Monitored Natural Attenuation (MNA). This article deals with currently available tools and approaches that can be used to support a decision to transition from active remediation to MNA. | |
<div style="float:right;margin:0 0 2em 2em;">__TOC__</div> | <div style="float:right;margin:0 0 2em 2em;">__TOC__</div> | ||
'''Related Article(s)''': | '''Related Article(s)''': | ||
| − | *[[ | + | *[Monitored Natural Attenuation (MNA)] |
| − | *[[ | + | *[Alternative Endpoints] |
| + | *[Source Zone Modeling] | ||
| + | *[Plume Response Modeling] | ||
| + | *[REMChlor-MD] | ||
| − | '''CONTRIBUTOR(S):''' [[Dr. | + | '''CONTRIBUTOR(S):''' [[Dr. John Wilson]] |
'''Key Resource(s)''': | '''Key Resource(s)''': | ||
| + | *[[media:2002-Newell-Calculation and Use of First-Order Rate Constants for Monitored Natural Attenuation Studies.pdf| Calculation and Use of First-Order Rate Constants for Monitored Natural Attenuation Studies.]]<ref name= "Newell2002">Newell, C.J., Rifai, H.S., Wilson, J.T., Connor, J.A., Aziz, J.A., Suarez, M.P., 2002. Calculation and use of first-order rate constants for monitored natural attenuation studies. 28p. EPA/540/S-02/500. [[media:2002-Newell-Calculation and Use of First-Order Rate Constants for Monitored Natural Attenuation Studies.pdf| Report.pdf]]</ref> | ||
| + | *[https://www.nas.cee.vt.edu/index.php Natural Attenuation Software (NAS) Version 2.3.3]<ref name= "Widdowson2008">Widdowson, M.A., Mendez, E., Chapelle, F.H., Casey, C.C., 2008. Natural Attenuation Software (NAS) Version 2.3.3. Virginia Polytechnic Institute and State University, the United States Geological Survey, and the United States Naval Facilities Engineering Command. </ref> | ||
| + | *[[media:2002-Aziz-Biochlor Natural Attenuation Decision Support System Vs 2.2.pdf| BIOCHLOR Natural Attenuation Support System, Version 2.2.]]<ref name= "Aziz2002">Aziz, C.E., Newell, C.J. and Gonzales, J.R., 2002. BIOCHLOR Natural Attenuation Decision Support System Version 2.2 User’s Manual Addendum. Groundwater Services, Inc., Houston, Texas for the Air Force Center for Environmental Excellence.[[media:2002-Aziz-Biochlor Natural Attenuation Decision Support System Vs 2.2.pdf| Report.pdf]]</ref> | ||
| + | |||
| + | ==Introduction== | ||
| + | Many active remedies are effective at treating higher concentrations of contaminants, but as the contaminant concentrations decrease, the rate of cleanup may slow until it is not significantly different from the rate of cleanup provided by the natural attenuation processes that occur at the site. The United States Environmental Protection Agency (USEPA 1999) <ref> U.S. Environmental Protection Agency (USEPA), 1999. Use of monitored natural attenuation at superfund, RCRA corrective action, and underground storage tank sites. [[media:1999 USEPA- Use of monitored natural attenuation at superfund.pdf| Report.pdf]]</ref> [1]allows the use of [[Monitored Natural Attenuation (MNA) | monitored natural attenuation (MNA)]] to attain the cleanup goals when the site-specific remediation objectives can be attained within a time frame that is reasonable compared to that offered by other more active methods. Many CERCLA<ref>U.S. Environmental Protection Agency (USEAP), 2019a. Summary of the Comprehensive Environmental Response, Compensation, and Liability Act (Superfund)</ref> and RCRA<ref>U.S. Environmental Protection Agency (USEPA), 2019b. Resource Conservation and Recovery Act (RCRA) Laws and Regulations</ref> sites take advantage of this policy. An active remedy is typically used initially to treat high concentrations of contaminants followed by MNA to treat the lower concentrations that remain. | ||
| + | |||
| + | Unfortunately, there is no well-established approach to determine when it is appropriate to discontinue the active remedy. This article reviews available tools and approaches to evaluate a transition to MNA. | ||
| + | The tools and approaches depend on calculations of rate constants for natural attenuation with distance in flowing groundwater or rate constants for attenuation over time in individual monitoring wells. The next section provides some background on first-order constants and how they are extracted from monitoring data. | ||
| + | |||
| + | ==Background on Rate Constants == | ||
| + | |||
| + | A general formula to describe the rate of a chemical reaction is: | ||
| + | |||
| + | :{| | ||
| + | |- | ||
| + | | '''Equation 1:''' || || <big>''r = k [C]</big><sup> m</sup>'' | ||
| + | |- | ||
| + | |where: | ||
| + | |- | ||
| + | | || ''r'' || is the rate of the reaction, | ||
| + | |- | ||
| + | | || ''k''|| is the rate constant, | ||
| + | |- | ||
| + | | || ''C''|| is the concentration of the chemical undergoing the reaction, and | ||
| + | |- | ||
| + | |the exponent || ''m''|| is the order of the reaction. | ||
| + | |} | ||
| + | |||
| + | When the rate of the reaction is proportional to the concentration of the contaminant, the value of ''m'' is 1. Therefore, the reaction is described as a first-order reaction, and the rate constant is described as a first-order rate constant. In Equation 1, concentration could go up or down, but ''k'' is a constant of proportionality for the rate of increase in concentration. The rate constant for attenuation is the negative of ''k''. If the rate of degradation is a fixed value regardless of concentration, the value of ''m'' is 0, and degradation is a zero-order process. | ||
| + | |||
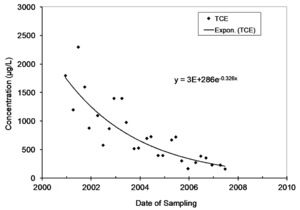
| + | Natural attenuation of concentrations over time in monitoring wells is frequently described by a first-order rate constant, and natural biological or abiotic degradation of contaminants in flowing groundwater is typically also described by a first-order rate constant. Figure 1 provides an example of monitoring data that is described by a first-order rate constant. | ||
| + | |||
| + | [[File:Wilson1w2Fig1.png|thumb|Figure 1. Attenuation of Trichloroethene (TCE) over time in a monitoring well at a site in Michigan. The concentration vs. time rate constant is 0.326 per year and largely represents the rate of the attenuation of the source of contaminants in the aquifer.]] | ||
| + | |||
| + | The rate constant for attenuation over time in a well and the rate constant for attenuation with distance along a flow path in an aquifer describe different situations that are controlled by different processes. ''Attenuation over time'' in a well is largely controlled by the rate of attenuation of the source of contamination in the aquifer. ''Attenuation with distance'' along a flow path includes attenuation of concentrations in the source along with contributions from biological degradation processes, abiotic degradation processes and hydrodynamic dispersion of the contaminated groundwater into clean groundwater<ref name= "Newell2002"/>. | ||
| + | |||
| + | The first-order rate constant for attenuation over time in a well is commonly referred to as ''k''<sub>point</sub><ref name= "Newell2002"/>. A chart in Microsoft EXCEL of concentration on date of sampling can be used to extract a value for ''k''<sub>point</sub>. Select the data, and then insert an exponential trend line and display the equation on the chart. The value of ''k''<sub>point</sub> can also be calculated in EXCEL using the Regression Analysis Tool in the Data Analysis Toolpak. Note that the rate constants extracted in EXCEL are constants for the rate of change, not the rate of attenuation. Take the negative of the rate of change to get ''k''<sub>point</sub>. In the example in Figure 1, the units of time on the X axis is years, and the value of ''k''<sub>point</sub> is 0.326 per year. | ||
| + | |||
| + | Attenuation versus distance rate coefficients describe a bulk attenuation rate of both degradation and dispersion processes. To extract values for rate constants for degradation alone, it is necessary to calibrate a groundwater flow and transport model to the data at the site. The model is calibrated with values for the hydrogeological properties of the aquifer (effective porosity, hydraulic gradient, hydraulic conductivity, hydrodynamic dispersion and the organic carbon content of the aquifer matrix). After the hydrogeological properties of the aquifer are fixed in the model, the most appropriate values for the degradation rate constants are the values that produce the best fit between the contaminant concentrations that are predicted by the model and the contaminant monitoring data at the site. | ||
| + | |||
| + | There are a number of reasons why natural attenuation processes are better described as first-order relationship instead of zero-order or some other order. The attenuation over time in a monitoring well tracks the attenuation over time of the source of contamination that sustains the plume<ref name= "Newell2002"/>. Sites go through a lifecycle, and attenuation of sources at mature sites is often a first-order process<ref>Sale, T., Newell, C., Stroo, H., Hinchee, R. and Johnson, P., 2008. Frequently asked questions regarding management of chlorinated solvents in soils and groundwater. Environmental Security Technology Certification Program Office (DoD), Arlington, VA (ER-200530). [[media: 2008-Sale-Frequently Asked Questions Regarding Management of Chlorinated Solvent in Soils and Groundwater.pdf| Report.pdf]]</ref>. If a chlorinated solvent site is mature, the contamination in the source area that was originally present as nonaqueous phase liquids (NAPL) has been redistributed and is now sequestered in a sorbed phase to aquifer solids or has diffused into non-transmissive portions of the aquifer matrix . Transfer of contaminants back into the more transmissive portions of the aquifer occurs by diffusion along a fixed path length, and the rate of transfer is controlled by the concentration of the contaminant remaining in the source material. Because the rate of transfer is proportional to the concentration of contaminant in the source material, attenuation of the source is a first-order process. These processes are discussed in more detail in [[Source Zone Modeling]]. | ||
| + | |||
| + | Degradation process are also usually first-order. Abiotic reactions are almost always first-order. Biodegradation reactions are zero-order at high concentrations because the enzymes are saturated with substrate but are first-order at lower concentrations that are typical of natural attenuation conditions in groundwater. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| − | |||
==References== | ==References== | ||
Revision as of 15:59, 17 January 2020
Many contaminated sites use active remedies such as pump-and-treat or in situ remediation to clean up impacted groundwater. Natural attenuation processes such as natural degradation or hydrodynamic dispersion also contribute to the cleanup. As remediation progresses, a point is often reached when the time required to reach the remedial objectives using the active remedy is roughly the same as the time required if the active remedy is shut down, and the continuing remediation of the site is provided by natural attenuation processes alone. From that point forward, the extra effort and expense of the active remedy provides no benefit over natural attenuation, and it may be appropriate to transition the site to Monitored Natural Attenuation (MNA). This article deals with currently available tools and approaches that can be used to support a decision to transition from active remediation to MNA.
Related Article(s):
- [Monitored Natural Attenuation (MNA)]
- [Alternative Endpoints]
- [Source Zone Modeling]
- [Plume Response Modeling]
- [REMChlor-MD]
CONTRIBUTOR(S): Dr. John Wilson
Key Resource(s):
- Calculation and Use of First-Order Rate Constants for Monitored Natural Attenuation Studies.[1]
- Natural Attenuation Software (NAS) Version 2.3.3[2]
- BIOCHLOR Natural Attenuation Support System, Version 2.2.[3]
Introduction
Many active remedies are effective at treating higher concentrations of contaminants, but as the contaminant concentrations decrease, the rate of cleanup may slow until it is not significantly different from the rate of cleanup provided by the natural attenuation processes that occur at the site. The United States Environmental Protection Agency (USEPA 1999) [4] [1]allows the use of monitored natural attenuation (MNA) to attain the cleanup goals when the site-specific remediation objectives can be attained within a time frame that is reasonable compared to that offered by other more active methods. Many CERCLA[5] and RCRA[6] sites take advantage of this policy. An active remedy is typically used initially to treat high concentrations of contaminants followed by MNA to treat the lower concentrations that remain.
Unfortunately, there is no well-established approach to determine when it is appropriate to discontinue the active remedy. This article reviews available tools and approaches to evaluate a transition to MNA. The tools and approaches depend on calculations of rate constants for natural attenuation with distance in flowing groundwater or rate constants for attenuation over time in individual monitoring wells. The next section provides some background on first-order constants and how they are extracted from monitoring data.
Background on Rate Constants
A general formula to describe the rate of a chemical reaction is:
Equation 1: r = k [C] m where: r is the rate of the reaction, k is the rate constant, C is the concentration of the chemical undergoing the reaction, and the exponent m is the order of the reaction.
When the rate of the reaction is proportional to the concentration of the contaminant, the value of m is 1. Therefore, the reaction is described as a first-order reaction, and the rate constant is described as a first-order rate constant. In Equation 1, concentration could go up or down, but k is a constant of proportionality for the rate of increase in concentration. The rate constant for attenuation is the negative of k. If the rate of degradation is a fixed value regardless of concentration, the value of m is 0, and degradation is a zero-order process.
Natural attenuation of concentrations over time in monitoring wells is frequently described by a first-order rate constant, and natural biological or abiotic degradation of contaminants in flowing groundwater is typically also described by a first-order rate constant. Figure 1 provides an example of monitoring data that is described by a first-order rate constant.
The rate constant for attenuation over time in a well and the rate constant for attenuation with distance along a flow path in an aquifer describe different situations that are controlled by different processes. Attenuation over time in a well is largely controlled by the rate of attenuation of the source of contamination in the aquifer. Attenuation with distance along a flow path includes attenuation of concentrations in the source along with contributions from biological degradation processes, abiotic degradation processes and hydrodynamic dispersion of the contaminated groundwater into clean groundwater[1].
The first-order rate constant for attenuation over time in a well is commonly referred to as kpoint[1]. A chart in Microsoft EXCEL of concentration on date of sampling can be used to extract a value for kpoint. Select the data, and then insert an exponential trend line and display the equation on the chart. The value of kpoint can also be calculated in EXCEL using the Regression Analysis Tool in the Data Analysis Toolpak. Note that the rate constants extracted in EXCEL are constants for the rate of change, not the rate of attenuation. Take the negative of the rate of change to get kpoint. In the example in Figure 1, the units of time on the X axis is years, and the value of kpoint is 0.326 per year.
Attenuation versus distance rate coefficients describe a bulk attenuation rate of both degradation and dispersion processes. To extract values for rate constants for degradation alone, it is necessary to calibrate a groundwater flow and transport model to the data at the site. The model is calibrated with values for the hydrogeological properties of the aquifer (effective porosity, hydraulic gradient, hydraulic conductivity, hydrodynamic dispersion and the organic carbon content of the aquifer matrix). After the hydrogeological properties of the aquifer are fixed in the model, the most appropriate values for the degradation rate constants are the values that produce the best fit between the contaminant concentrations that are predicted by the model and the contaminant monitoring data at the site.
There are a number of reasons why natural attenuation processes are better described as first-order relationship instead of zero-order or some other order. The attenuation over time in a monitoring well tracks the attenuation over time of the source of contamination that sustains the plume[1]. Sites go through a lifecycle, and attenuation of sources at mature sites is often a first-order process[7]. If a chlorinated solvent site is mature, the contamination in the source area that was originally present as nonaqueous phase liquids (NAPL) has been redistributed and is now sequestered in a sorbed phase to aquifer solids or has diffused into non-transmissive portions of the aquifer matrix . Transfer of contaminants back into the more transmissive portions of the aquifer occurs by diffusion along a fixed path length, and the rate of transfer is controlled by the concentration of the contaminant remaining in the source material. Because the rate of transfer is proportional to the concentration of contaminant in the source material, attenuation of the source is a first-order process. These processes are discussed in more detail in Source Zone Modeling.
Degradation process are also usually first-order. Abiotic reactions are almost always first-order. Biodegradation reactions are zero-order at high concentrations because the enzymes are saturated with substrate but are first-order at lower concentrations that are typical of natural attenuation conditions in groundwater.
References
- ^ 1.0 1.1 1.2 1.3 Newell, C.J., Rifai, H.S., Wilson, J.T., Connor, J.A., Aziz, J.A., Suarez, M.P., 2002. Calculation and use of first-order rate constants for monitored natural attenuation studies. 28p. EPA/540/S-02/500. Report.pdf
- ^ Widdowson, M.A., Mendez, E., Chapelle, F.H., Casey, C.C., 2008. Natural Attenuation Software (NAS) Version 2.3.3. Virginia Polytechnic Institute and State University, the United States Geological Survey, and the United States Naval Facilities Engineering Command.
- ^ Aziz, C.E., Newell, C.J. and Gonzales, J.R., 2002. BIOCHLOR Natural Attenuation Decision Support System Version 2.2 User’s Manual Addendum. Groundwater Services, Inc., Houston, Texas for the Air Force Center for Environmental Excellence. Report.pdf
- ^ U.S. Environmental Protection Agency (USEPA), 1999. Use of monitored natural attenuation at superfund, RCRA corrective action, and underground storage tank sites. Report.pdf
- ^ U.S. Environmental Protection Agency (USEAP), 2019a. Summary of the Comprehensive Environmental Response, Compensation, and Liability Act (Superfund)
- ^ U.S. Environmental Protection Agency (USEPA), 2019b. Resource Conservation and Recovery Act (RCRA) Laws and Regulations
- ^ Sale, T., Newell, C., Stroo, H., Hinchee, R. and Johnson, P., 2008. Frequently asked questions regarding management of chlorinated solvents in soils and groundwater. Environmental Security Technology Certification Program Office (DoD), Arlington, VA (ER-200530). Report.pdf