Difference between revisions of "User:Debra Tabron/sandbox"
Debra Tabron (talk | contribs) |
Debra Tabron (talk | contribs) |
||
| Line 27: | Line 27: | ||
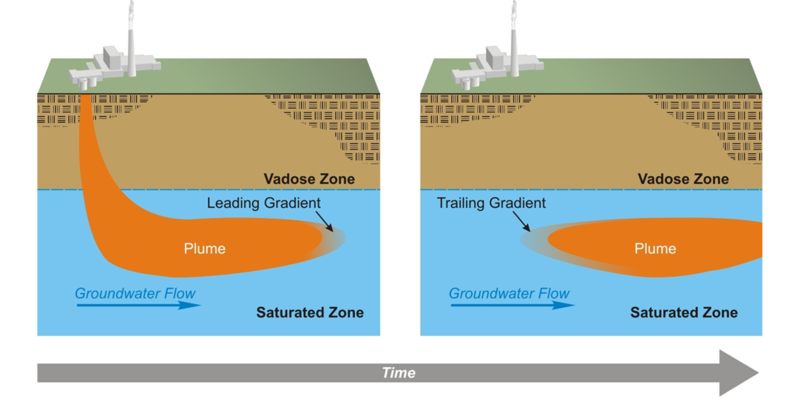
Consider the evolution of a contamination plume in an aquifer (Fig. 1). As contamination enters the aquifer, a '''geochemical gradient''' forms at the leading edge of the plume<ref name="Truex2011"/>. This leading gradient may simply be a concentration gradient of the contaminant, where the concentration is higher on the side of the plume opposite of the direction of flow. In many cases, the leading gradient also includes gradients in concentrations of other constituents associated with the contaminant source. For example, contamination plumes often have different pH, oxidation-reduction potential, or ionic strength than the native groundwater. In any event, reactions tend to occur that counter the geochemical gradient and can cause adsorption of the contaminant to aquifer mineral surfaces or precipitation of the contaminant. | Consider the evolution of a contamination plume in an aquifer (Fig. 1). As contamination enters the aquifer, a '''geochemical gradient''' forms at the leading edge of the plume<ref name="Truex2011"/>. This leading gradient may simply be a concentration gradient of the contaminant, where the concentration is higher on the side of the plume opposite of the direction of flow. In many cases, the leading gradient also includes gradients in concentrations of other constituents associated with the contaminant source. For example, contamination plumes often have different pH, oxidation-reduction potential, or ionic strength than the native groundwater. In any event, reactions tend to occur that counter the geochemical gradient and can cause adsorption of the contaminant to aquifer mineral surfaces or precipitation of the contaminant. | ||
| + | [[File:Denham-Article 3-Figure 1.PNG|800px|center]] | ||
| + | <div align="center">Figure 1. Typical contaminant plume evolution in an aquifer showing leading and trailing gradients<ref name= "ITRC2010"/>.</div> | ||
| + | The rate of the reactions and the supply of reactants in the aquifer at the leading gradient of a contamination plume influence the rate at which the contamination plume moves. The supply of reactants includes the concentration of reactive minerals and the concentration of available adsorption sites and is called the aquifer attenuation capacity. Once the source of contamination has been eliminated, the migration rate of the leading edge or gradient of the contaminant plume will be zero or near zero if rates of adsorption or precipitation are fast relative to groundwater flow and attenuation capacity is sufficient to react with all the contamination in the aquifer<ref name= "USEPA2007V1"/><ref name="Truex2011"/><ref>Bekins, B., Rittmann, B.E. and MacDonald, J.A., 2001. Natural attenuation strategy for groundwater cleanup focuses on demonstrating cause and effect. Eos, Transactions American Geophysical Union, 82(5), pp.53-58. [http://dx.doi.org/10.1029/01eo00028 doi: 10.1029/01EO00028]</ref>. These conditions are necessary for MNA to be a viable remedy for metal and metalloid contaminants. | ||
| + | Another necessary condition for MNA viability is that the flux of contaminants from the zone of attenuation be too low now and in the future to present a hazard. Just as there is a leading gradient of a contamination plume, there is also a trailing gradient (Fig. 1) caused by influx of native (“clean”) groundwater into the attenuation zone. If reactions in the trailing gradient cause contaminants to desorb or dissolve at a rate that produces a flux of contaminants from the attenuation zone that presents a hazard, then MNA is not a viable remedy. | ||
| + | |||
| + | Characterization and study of reactions in the leading geochemical gradient of a contaminant plume identify attenuation mechanisms and quantify attenuation capacity. Characterization and study of reactions in the trailing geochemical gradient determine whether attenuation will be long-lived enough for MNA to be viable as a remedy. | ||
| + | |||
| + | == Guidance on MNA as a Remedy== | ||
| + | In 2015, the U.S. Environmental Protection Agency (USEPA) published guidance for use of MNA for inorganic contaminants<ref name= "EPA1999"/>. This approach and criteria for using MNA integrates the framework of guidance issued in 1999 on using MNA for organic contaminants<ref name="EPA2015"/> with the technical approaches for inorganic contaminants issued in 2007<ref name= "USEPA2007V1"/>. Companion documents exist<ref name= "USEPA2007V1"/> that provide characterization and technical guidance to demonstrating MNA for specific nonradioactive inorganic contaminants<ref name="USEPA2007a"/> and radionuclides<ref name="USEPA2010"/>. In addition, the Interstate Technology and Regulatory Council published a “Technical/Regulatory Guidance” document that provides a decision framework for applying the USEPA guidance, as well as providing the perspective of some state regulatory agencies and stakeholders<ref name= "ITRC2010"/>. | ||
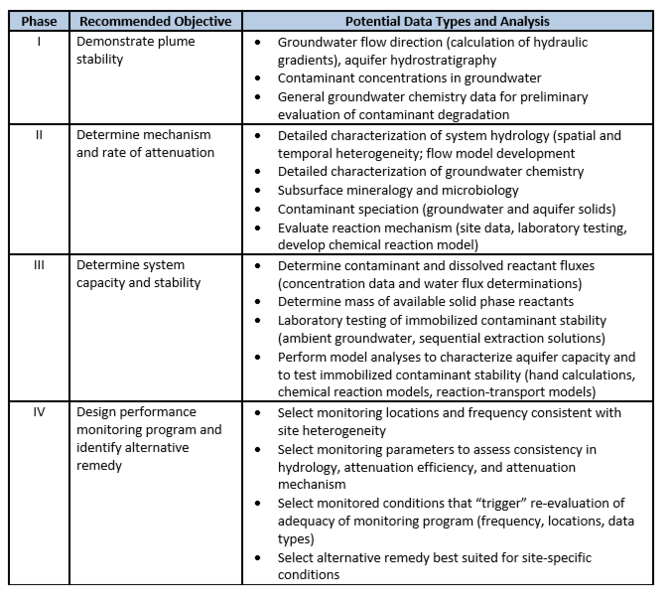
| + | The basis for demonstrating MNA for inorganic contaminants, such as metals and metalloids, is a tiered four-phase strategy (outlined in Table 1). The use of MNA for inorganic contaminants is predicated on two conditions: | ||
| + | |||
| + | #The source of contamination has been contained or eliminated | ||
| + | #The contamination plume is not expanding | ||
| + | |||
| + | Furthermore, the published guidance for use of MNA for inorganic contaminants<ref name= "EPA1999"/> discourages the use of dispersion as a primary attenuation mechanism as follows<ref name="EPA2015"/>:<br /> | ||
| + | '' “…dilution and dispersion generally are not appropriate as primary MNA mechanisms because they reduce concentrations through dispersal of contaminant mass rather than destruction or immobilization of contaminant mass.”'' | ||
| + | |||
| + | Throughout these documents it is repeatedly emphasized that MNA is not a “do nothing” remedy. This is certainly the case, given the burden of proof required to successfully demonstrate MNA as a viable remedy for metal and metalloid contamination. | ||
| + | |||
| + | [[File:Denham-Article 3-Table 1.PNG|671px|thumbnail|center|Table 1. Tiered four-phase approach to demonstrating MNA for inorganic compounds<ref name= "EPA1999"/>.]] | ||
| + | |||
| + | ==The Scenarios Approach to Attenuation-Based Remedies== | ||
| + | In 2011, the U.S. Department of Energy published the “Scenarios Approach to Attenuation‐Based Remedies for Inorganic and Radionuclide Contaminants”<ref name="Truex2011"/> to serve as a technical resource to guide waste site owners, regulators, stakeholders, or other interested parties through the process of evaluating attenuation-based remedies for sites contaminated with inorganic or radionuclide contaminants. A structured approach is provided where: | ||
| + | |||
| + | ''The scenarios approach exploits important traits that waste sites may have in common that allow them to be grouped into six categories or scenarios. The common traits of each scenario are parameters or characteristics that are important to attenuation of inorganic and radionuclide contaminants. A single waste site may host multiple scenarios, each occurring in different segments of a contaminant plume or predicted to occur at different points in time during the evolution of the waste site''<ref name="Truex2011"/>. | ||
| + | |||
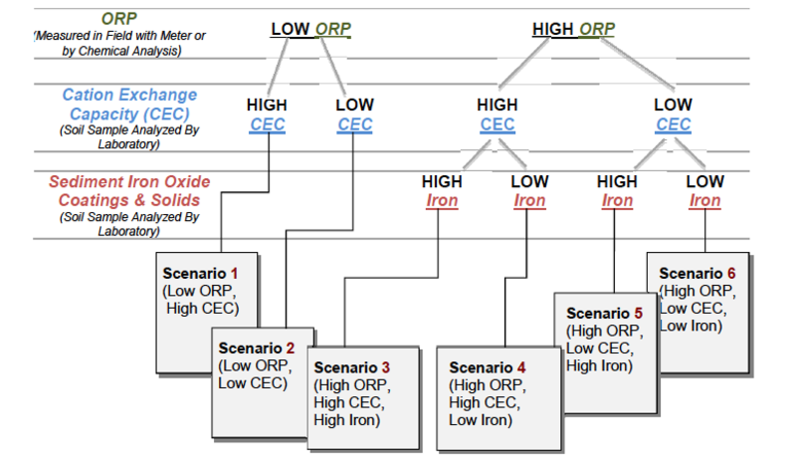
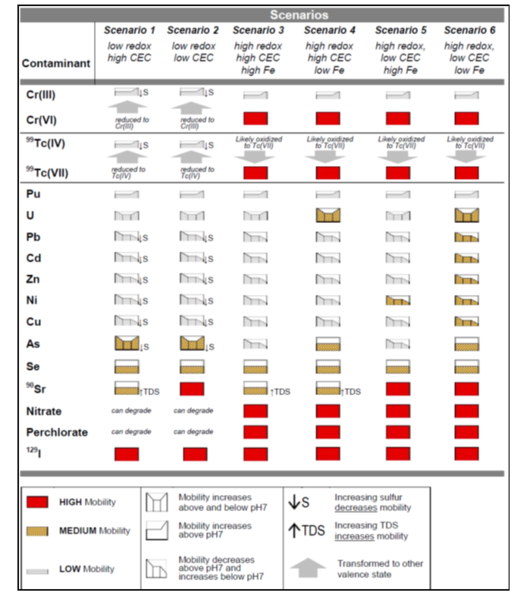
| + | There are six scenarios that are a function of three primary factors: oxidation/reduction potential (ORP); cation exchange capacity (CEC), and sediment iron oxide coatings and solids (Table 2). There are three primary factors and three secondary factors (pH, sulfur/sulfide, and total dissolved solids) that combine with the six scenarios to provide a semi-qualitative indicator of mobility (i.e., low mobility is defined as a retardation factor of 1000 or more) (Fig. 2). | ||
| + | |||
| + | [[File:Denham-Article 3-Table 2.PNG|800 px|center]] | ||
| + | <div align="center">Table 2. Six Scenarios for Evaluating Inorganic Monitored Natural Attenuation<ref name="Truex2011"/>.</div> | ||
| + | |||
| + | |||
| + | [[File:Denham-Article 3-Figure 2.PNG|524px|thumbnail|center|Figure 2. Summary of inorganic contaminant mobility for 4 < pH < 9 for six scenarios<ref name="Truex2011"/>.]] | ||
==References== | ==References== | ||
Revision as of 17:56, 23 January 2017
At some sites Monitored Natural Attenuation (MNA) can manage metals and metalloid contaminants (“metals”) if the contaminants can be safely held in place on aquifer materials by sorption and/or precipitation processes. The degree and permanence of precipitation and adsorption can be evaluated using the concept of geochemical gradients, where key aquifer properties such as pH, redox potential, and ionic strength are different within the plume and can change over time as the plume moves through the subsurface. The U.S. Environmental Protection Agency (USEPA) developed an extensive three-volume guidance document and a Directive that can be used to determine if MNA can be applied at a site with metal contaminants in groundwater[1][2][3][4]. Using technical aspects of this guidance document as a foundation, we also overview a “Scenarios Approach”, developed by the U.S. Department of Energy for evaluating MNA for metals in groundwater that shows the mobility chart for a number of metals for six different geochemical scenarios[5].
Related Article(s):
CONTRIBUTOR(S): Dr. Miles Denham and Dr. Charles Newell, P.E.
Key Resource(s):
- A Decision Framework for Applying Monitored Natural Attenuation Processes to Metals and Radionuclides[6]
- Use of Monitored Natural Attenuation At Superfund, RCRA Corrective Action, And Underground Storage Tank Sites[7]
Introduction
Monitored natural attenuation (MNA) of metal and metalloid contaminants in groundwater is a remediation strategy that relies on natural processes occurring in an aquifer that minimize risk to human health and the environment by attenuating contaminant migration from their source to a compliance point such as a drinking water well or property boundary. With the exception of short-lived radionuclides, metals and metalloids are not destroyed by natural attenuation processes and will remain sequestered in the aquifer when sufficiently attenuated. Therefore, gaining acceptance of MNA as a remedy for metal and metalloid contamination requires demonstrating, to an acceptable degree of uncertainty, that the contaminants will remain in place and pose a minimal risk for decades to centuries, depending on the contaminant.
Attenuation of Metals and Metalloids
Natural attenuation processes of metals and metalloids that occur in aquifers are adsorption, precipitation, radioactive decay, and dispersion. Adsorption and precipitation limit the mobility of metals and metalloids by causing them to partition from groundwater to solid phases. Dispersion dilutes the concentration of contaminants, rather than limiting their mobility. Radioactive decay applies to only those radionuclides that have short enough half-lives to prevent their migration to compliance points. “Short enough” varies with the contamination scenario. Radioactive decay may be sufficient to achieve successful MNA if the groundwater travel time to the compliance point is much longer than the half-life of the radionuclide.
| Geochemical Gradients |
| Geochemical gradients are the spatial variations in geochemical conditions created by waste disposal or other phenomena. Geochemical gradients can evolve over time as geochemical conditions change; for instance, as neutral pH water displaces low pH water. When present, geochemical gradients can strongly affect contaminant mobility and thus identification and characterization of geochemical of gradients allows the contaminant attenuation-affecting conditions of a site to be projected into the future[5]. |
Demonstrating that adsorption and/or precipitation are sufficiently limiting the mobility of a contaminant metal or metalloid is the strongest evidence that MNA is an appropriate remedy. Adsorption and precipitation may occur when a metal or metalloid enters groundwater because the contaminant, and often the composition of fluids carrying the contaminant, cause perturbations of the near steady-state condition of the groundwater system. This promotes reactions that tend to return the groundwater system toward its original state and these often result in contaminant adsorption, precipitation, or both.
Consider the evolution of a contamination plume in an aquifer (Fig. 1). As contamination enters the aquifer, a geochemical gradient forms at the leading edge of the plume[5]. This leading gradient may simply be a concentration gradient of the contaminant, where the concentration is higher on the side of the plume opposite of the direction of flow. In many cases, the leading gradient also includes gradients in concentrations of other constituents associated with the contaminant source. For example, contamination plumes often have different pH, oxidation-reduction potential, or ionic strength than the native groundwater. In any event, reactions tend to occur that counter the geochemical gradient and can cause adsorption of the contaminant to aquifer mineral surfaces or precipitation of the contaminant.
The rate of the reactions and the supply of reactants in the aquifer at the leading gradient of a contamination plume influence the rate at which the contamination plume moves. The supply of reactants includes the concentration of reactive minerals and the concentration of available adsorption sites and is called the aquifer attenuation capacity. Once the source of contamination has been eliminated, the migration rate of the leading edge or gradient of the contaminant plume will be zero or near zero if rates of adsorption or precipitation are fast relative to groundwater flow and attenuation capacity is sufficient to react with all the contamination in the aquifer[2][5][8]. These conditions are necessary for MNA to be a viable remedy for metal and metalloid contaminants.
Another necessary condition for MNA viability is that the flux of contaminants from the zone of attenuation be too low now and in the future to present a hazard. Just as there is a leading gradient of a contamination plume, there is also a trailing gradient (Fig. 1) caused by influx of native (“clean”) groundwater into the attenuation zone. If reactions in the trailing gradient cause contaminants to desorb or dissolve at a rate that produces a flux of contaminants from the attenuation zone that presents a hazard, then MNA is not a viable remedy.
Characterization and study of reactions in the leading geochemical gradient of a contaminant plume identify attenuation mechanisms and quantify attenuation capacity. Characterization and study of reactions in the trailing geochemical gradient determine whether attenuation will be long-lived enough for MNA to be viable as a remedy.
Guidance on MNA as a Remedy
In 2015, the U.S. Environmental Protection Agency (USEPA) published guidance for use of MNA for inorganic contaminants[7]. This approach and criteria for using MNA integrates the framework of guidance issued in 1999 on using MNA for organic contaminants[1] with the technical approaches for inorganic contaminants issued in 2007[2]. Companion documents exist[2] that provide characterization and technical guidance to demonstrating MNA for specific nonradioactive inorganic contaminants[3] and radionuclides[4]. In addition, the Interstate Technology and Regulatory Council published a “Technical/Regulatory Guidance” document that provides a decision framework for applying the USEPA guidance, as well as providing the perspective of some state regulatory agencies and stakeholders[6]. The basis for demonstrating MNA for inorganic contaminants, such as metals and metalloids, is a tiered four-phase strategy (outlined in Table 1). The use of MNA for inorganic contaminants is predicated on two conditions:
- The source of contamination has been contained or eliminated
- The contamination plume is not expanding
Furthermore, the published guidance for use of MNA for inorganic contaminants[7] discourages the use of dispersion as a primary attenuation mechanism as follows[1]:
“…dilution and dispersion generally are not appropriate as primary MNA mechanisms because they reduce concentrations through dispersal of contaminant mass rather than destruction or immobilization of contaminant mass.”
Throughout these documents it is repeatedly emphasized that MNA is not a “do nothing” remedy. This is certainly the case, given the burden of proof required to successfully demonstrate MNA as a viable remedy for metal and metalloid contamination.
The Scenarios Approach to Attenuation-Based Remedies
In 2011, the U.S. Department of Energy published the “Scenarios Approach to Attenuation‐Based Remedies for Inorganic and Radionuclide Contaminants”[5] to serve as a technical resource to guide waste site owners, regulators, stakeholders, or other interested parties through the process of evaluating attenuation-based remedies for sites contaminated with inorganic or radionuclide contaminants. A structured approach is provided where:
The scenarios approach exploits important traits that waste sites may have in common that allow them to be grouped into six categories or scenarios. The common traits of each scenario are parameters or characteristics that are important to attenuation of inorganic and radionuclide contaminants. A single waste site may host multiple scenarios, each occurring in different segments of a contaminant plume or predicted to occur at different points in time during the evolution of the waste site[5].
There are six scenarios that are a function of three primary factors: oxidation/reduction potential (ORP); cation exchange capacity (CEC), and sediment iron oxide coatings and solids (Table 2). There are three primary factors and three secondary factors (pH, sulfur/sulfide, and total dissolved solids) that combine with the six scenarios to provide a semi-qualitative indicator of mobility (i.e., low mobility is defined as a retardation factor of 1000 or more) (Fig. 2).
References
- ^ 1.0 1.1 1.2 U.S. Environmental Protection Agency (USEPA), 2015. Use of Monitored Natural Attenuation for Inorganic contaminants in Groundwater at Superfund Sites. Directive 9283.1-36, Office of Solid Waste and Emergency Response, United States Environmental Protection Agency. Report.pdf
- ^ 2.0 2.1 2.2 2.3 United States Environmental Protection Agency (USEPA), 2007. Monitored natural attenuation of inorganic contaminants in groundwater, Volume 1 Technical basis for assessment, Edited by R.G. Ford, R.T. Wilkin, and R.W. Puls. U.S. Environmental Protection Agency, EPA/600/R-07/139. Report pdf
- ^ 3.0 3.1 U.S. Environmental Protection Agency (USEPA), 2007. Monitored natural attenuation of inorganic contaminants in groundwater, Volume 2 Assessment for Non-Radionuclides Including Arsenic, Cadmium, Chromium, Copper, Lead, Nickel, Nitrate, Perchlorate, and Selenium, Edited by R.G. Ford, R.T. Wilkin, and R.W. Puls. EPA/600/R-07/140. Report pdf
- ^ 4.0 4.1 U.S. Environmental Protection Agency (USEPA), 2010. Monitored natural attenuation of inorganic contaminants in groundwater, Volume 3 Assessment for Radionuclides Including Tritium, Radon, Strontium, Technetium, Uranium, Iodine, Radium, Thorium, Cesium, and Plutonium-Americium, Edited by R.G. Ford and R.T. Wilkin. U.S. Environmental Protection Agency, EPA/600/R-10/093. Report pdf
- ^ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 Truex, M., Brady, P., Newell, C.J., Rysz, M., Denham, M., Vangelas, K. 2011. The scenarios approach to attenuation-based remedies for inorganic and radionuclide contaminants. Savannah-River National Laboratory U.S. Department of Energy. Report pdf
- ^ 6.0 6.1 6.2 ITRC, 2010. A decision framework for applying monitored natural attenuation processes to metals and radionuclides, Interstate Technology and Regulatory Council, Technical/Regulatory Guidance AMPR-1. Report pdf
- ^ 7.0 7.1 7.2 7.3 U.S. Environmental Protection Agency (USEPA), 1999. Use of monitored natural attenuation at superfund, RCRA corrective action, and underground storage tank sites. Report.pdf
- ^ Bekins, B., Rittmann, B.E. and MacDonald, J.A., 2001. Natural attenuation strategy for groundwater cleanup focuses on demonstrating cause and effect. Eos, Transactions American Geophysical Union, 82(5), pp.53-58. doi: 10.1029/01EO00028