User:Debra Tabron/sandbox
Acids are produced during Enhanced Reductive Dechlorination (ERD) which can cause pH to drop, inhibiting treatment. Alkaline materials including hydroxides and carbonates are sometimes added to the aquifer during ERD to maintain a pH of greater than 6, improving bioremediation performance. This article describes a simplified approach and a spreadsheet-based design tool for estimating the total amount of base required to achieve a specified target pH at the end of the treatment period, once all reactions have gone to completion. To prevent overshoot and excessively high pH, users typically add a fraction of the total base required in several increments spread over time.
Related Article(s):
- pH Buffering in Aquifers
- Low pH Inhibition of Reductive Dechlorination
- Bioremediation – Anaerobic
- Chlorinated Solvents
CONTRIBUTOR(S): Dr. Robert Borden, P.E.
Key Resource(s):
- Spreadsheet-based Design Tool -- Base Addition for ERD
- Post-Remediation Evaluation of EVO Treatment: How Can we improve performance[1]
Introduction
Aquifer pH lower than 6 can reduce the efficiency of enhanced reductive dechlorination (ERD) and other in situ remediation processes. This article describes a simplified approach using a spreadsheet-based design tool for estimating the total amount of base required to achieve a specified target pH at the end of the treatment period, once all reactions have gone to completion. This approach requires simplification of several important processes controlling subsurface pH, and is only appropriate for developing initial estimates of the total amount of base required. In practice, aquifer pH should be monitored during ERD and periodically adjusted to maintain conditions for optimal microbial growth and contaminant degradation. Early in the bioremediation process, reactions will not have gone to completion and less base will be required to maintain the target pH. To prevent overshoot and excessively high pH, users typically add a fraction of the total base required in several increments spread over time.
Background Aquifer pH
In humid areas, rainfall combined with carbonic acid produced in the soil leaches out base cations (Na+, K+, Ca2+, Mg2+) gradually acidifying the soil. Figure 1 shows soil pH in the contiguous United States.
(FIGURE 1 HERE) NEED TO REDUCE FILE SIZE
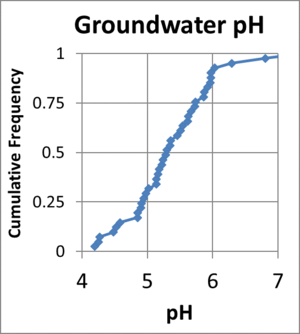
Groundwater pH is influenced by soil pH, but also by other factors. As acidic water infiltrates through the soil profile, some of the acidity may be neutralized by dissolution of soil and aquifer minerals. Silicate minerals including feldspars and micas can hydrolyze over time, consuming acid, and releasing dissolved cations (Na+, K+, Ca2+, Mg2+). However, these weathering reactions are slow, and are often not sufficient to prevent pH declines during ERD. Figure 2 shows a cumulative frequency distribution of groundwater pH at a site in eastern North Carolina. Average soil pH at this site is approximately 5. The vadose zone and surficial aquifer at the site are predominantly quartz sand and weathered clays, so weathering processes provide minimal buffering capacity. 90% of the groundwater pH measurements were between 4.5 and 6.0 indicating low soil pH at this site was a reasonable predictor of acidic groundwater. At other sites containing carbonate minerals or relatively young rocks, mineral dissolution often results in greater buffering.
Groundwater Acidity
Acidity is the amount of base required to neutralize acids present in a water sample. Since various acids can disassociate to different extents, two solutions with the same pH can have different acidities (see pH Buffering in Aquifers). In most cases, the acidity of the background groundwater is the sum of acidity from strong mineral acids (phosphoric, nitric, sulfuric, and hydrochloric acids), organic acids, and dissolved carbon dioxide. Mineral acidity (also referred to as methyl orange acidity) is determined by titrating a sample with a strong base to pH=3.7 (Standard Method 2310, AWPA 2016). If the initial pH is greater than 3.7, mineral acidity is zero. The spreadsheet-based design tool presented here uses this measurement to account for the base demand of any strong acids already present in the aquifer. Total acidity (commonly referred to as phenolphthalein acidity) should not be used to calculate the amount of base required because the titration endpoint of this parameter is pH 8.3, which will over-estimate the base requirement for optimal ERD.
Under low pH conditions, organic volatile fatty acids (acetic, propionic, etc.) can accumulate and contribute to acidity. However, in background groundwater, organic acid concentrations are generally low. In the design tool, acidity from background organic acids is assumed to be negligible.
In theory, acidity from dissolved carbon dioxide can be measured using a modification of Standard Method 2310[2] in which the sample is titrated to a target pH using a strong base. When using this approach, special precautions should be taken to prevent any pH shift due to degassing of dissolved carbon dioxide from the sample during transport to the laboratory or during analysis of the sample. In the design tool, acidity from background dissolved carbon dioxide is instead calculated from the dissolved inorganic carbon concentration (DIC) and background pH. This eliminates the problem of pH shift due to dissolved carbon dioxide degassing. Commercial laboratories can measure DIC using a modification of the Total Organic Carbon (TOC) procedure.
Aquifer Buffering Capacity
An aquifer’s buffering capacity is the resistance to pH change and is primarily due to two processes: (1) buffering by dissolved and solid carbonates; and (2) surface complexation and/or ion exchange reactions on mineral surfaces. While mineral weathering processes do influence long-term pH changes, weathering reactions are often too slow to prevent pH declines due to HCl and CO2 production during ERD.
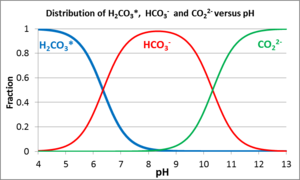
Buffering by Dissolved and Solid Carbonates In natural aqueous systems, pH buffers are predominantly weak acid anions that easily bind and release hydrogen ions. The most common are the weak acid anions produced by dissolved CO2 [3][4]. When CO2 dissolves in water, some CO2 combines with H2O forming carbonic acid (H2CO3). For convenience, the sum of dissolved CO2 and H2CO3 is often written as H2CO3*. H2CO3*can then disassociate releasing a bicarbonate ion (HCO3-) and one H+ or a carbonate ion (CO32-) and two H+ by the following[5]:
Figure 3 shows the relative distribution of these solutes as a function of pH. The reactions are reversible so that (a) an influx of acid will cause the HCO3- and CO32- ions to protonate, consuming the acid, or (b) an influx of base will cause dissociation (deprotonation) of H2CO3* and the bicarbonate ion to consume the base. The maximum resistance to pH change (buffering capacity) occurs when the pH is equal to the dissociation constant of either carbonic acid (pH = 6.3 @ 25°C) or of the bicarbonate ion (pH = 10.3 @ 25°C). The buffering capacity of groundwater is measured with an alkalinity titration[6][2]. The units of alkalinity can be given as milliequivalents (meq) per liter, but are often reported as mg CaCO3/L (milligrams of CaCO3 per liter) using the following equation:
For a system open to a large reservoir of carbon dioxide (e.g. the atmosphere), the buffering capacity (assuming no soluble minerals are present) is a function of the partial pressure of carbon dioxide in the reservoir. Near the water table, groundwater is open to the atmosphere and can release excess dissolved CO2 gas if its partial pressure is greater than the reservoir’s, essentially stripping some acid out of the groundwater by partially reversing the above reactions.
In a closed system (e.g. below the water table), the buffering capacity is a function of the total dissolved carbonates (H2CO3*, HCO3-, and CO3-2). When solid carbonate minerals are present (CaCO3, MgCO3, CaMg(CO3)2, FeCO3), carbonate mineral dissolution can limit pH declines caused by strong acids (e.g. HCl). For example, in an aquifer containing solid CaCO3, the ambient groundwater is saturated with CaCO3 (s). When HCl is produced by ERD, groundwater pH declines, and dissolved CO32- also declines as the carbonate ion is protonated by the added H+ ions causing an increase in HCO3- and/or H2CO3* (see Figure 3). The groundwater is now under-saturated and CaCO3 will dissolve, consuming H+ by the following reaction:
However, the solubility of carbonate minerals is relatively low, so solid carbonates are much less effective in limiting pH declines due to CO2 production in the saturated zone. Below the water table, CO2 may not degas, causing a buildup of HCO3-. Geochemical modeling[7] indicates that the aquifer can become supersaturated with CaCO3 during ERD, causing carbonate dissolution to stop. As additional CO2 is produced, CaCO3 (s) will precipitate producing H+ by the following reaction:
Reaction 4
Surface Complexation and Ion Exchange Reactions H+ sorption to Fe and Al oxyhydroxides and clay minerals through surface complexation and ion exchange reactions can have a major impact on pH. H+ adsorbs strongly to some mineral surfaces, which can accumulate large amounts of H+ as the solution pH declines and release the H+ back to solution as the pH rises[8][9]. This strong buffer can reduce the pH decline in many systems, but can also greatly increase the amount of base required to increase aquifer pH.
Estimating Aquifer Buffering Capacity When carbonate minerals are present, geochemical models[7] are needed to determine the amount of buffering provided by carbonate mineral dissolution. However, in many naturally low pH aquifers, carbonate minerals are absent and the extent of buffering can be estimated by adding a strong base to the aquifer solids, equilibrating for several days, and measuring the resulting pH. These buffering curves are typically linear in the pH range of 4.5 to 6.5[10][11].[12] recommend carrying out the titrations in a uniform ionic strength solution, most commonly 0.01 M CaCl2.
Figure 4 shows the measured final pH versus meq of OH- added per kg of dry aquifer material. Incremental base addition results in a nearly linear increase in pH. The slopes of these curves were estimated by linear regression. pH buffer capacity (pHBC) is calculated as the inverse of the slope and is reported in units of milliequivalents per kilogram (meq/kg) per pH unit.
References
- ^ Borden, R.C., 2017. Post-Remediation Evaluation of EVO Treatment: How Can We Improve Performance. Environmental Security Technology Certification Program, Alexandria, VA. ER-201581 Report.pdf
- ^ 2.0 2.1 American Public Health Association, American Water Works Association and Water Environment Federation (APWA, AWWA, and WEF), 2016. Standard Method 2320 Alkalinity, Standard methods for the examination of water and wastewater. Report.pdf
- ^ Langmuir, D., 1997, Aqueous Environmental Geochemistry. Prentice-Hall, Inc. Upper Saddle River, NJ ISBN: 978-0023674129.
- ^ Drever, J.I., The Geochemistry of Natural Waters: Surface and Groundwater Environments. Prentice-Hall, Inc., ISBN 0132727900.
- ^ Stumm, W. and Morgan, J.J., 1996. Aquatic chemistry; an introduction emphasizing chemical equilibria in natural waters. ISBN-13: 978-0471091738 and ISBN-10: 0471091731
- ^ U.S. Geological Survey, 2015. National field manual for the collection of water-quality field data, Alkalinity and acid neutralizing capacity. US Geological Survey Techniques of Water-Resources Investigations, book 9, chap. A6., sec. 6.6. [[media:USGS-2015-Natl_Field_Manual.pdf| Report pdf]
- ^ 7.0 7.1 Robinson, C., Barry, D.A., McCarty, P.L., Gerhard, J.I. and Kouznetsova, I., 2009. pH control for enhanced reductive bioremediation of chlorinated solvent source zones. Science of the Total Environment, 407(16), pp.4560-4573. doi:10.1016/j.scitotenv.2009.03.029
- ^ Davis, J.A. and Kent, D.B., 1990. Surface complexation modeling in aqueous geochemistry. Reviews in Mineralogy and Geochemistry, 23(1), pp.177-260. doi: 10.1021/es980312q
- ^ Davis, J.A., Coston, J.A., Kent, D.B. and Fuller, C.C., 1998. Application of the surface complexation concept to complex mineral assemblages. Environmental Science & Technology, 32(19), pp.2820-2828. doi: 10.1021/es980312q
- ^ Magdoff, F.R. and Bartlett, R.J., 1985. Soil pH Buffering Revisited 1. Soil Science Society of America Journal, 49(1), pp.145-148. doi: 10.2136/sssaj1985.03615995004900010029x
- ^ Liu, M., Kissel, D.E., Cabrera, M.L. and Vendrell, P.F., 2005. Soil lime requirement by direct titration with a single addition of calcium hydroxide. Soil Science Society of America Journal, 69(2), pp.522-530. doi:10.2136/sssaj2005.0522
- ^ Aitken, R.L. and Moody, P.W., 1994. The effect of valence and ionic-strength on the measurement of pH buffer capacity. Soil Research, 32(5), pp.975-984. doi: 10.1071/SR9940975