Difference between revisions of "User:Jhurley/sandbox"
(→Thermal Treatment) |
(→Stabilization) |
||
| Line 30: | Line 30: | ||
===Stabilization=== | ===Stabilization=== | ||
| − | Various amendments have been manufactured to sorb PFAS to reduce leaching from soil. Although this is a non-destructive approach, stabilization can reduce mass flux from a source area or allow soils to be placed in landfills with reduced potential for leaching. Amendments sorb PFAS through hydrophobic and electrostatic interactions and are applied to soil through ''in situ'' soil mixing or ''ex situ'' stabilization. Effectiveness of amendments varies depending on site conditions, PFAS types present, and mixing conditions<ref name="ITRCwNs2020"/>. Good results have been observed in bench and field scale tests with a variety of cationic clays (natural or chemically modified) and zeolites<ref name="OchoaHerrera2008">Ochoa-Herrera, V., and Sierra-Alvarez, R., 2008. Removal of perfluorinated surfactants by sorption onto granular activated carbon, zeolites and sludge. Chemosphere, 72(10), pp. 1588-1593. [https://doi.org/10.1016/j.chemosphere.2008.04.029 DOI: 10.1016/j.chemosphere.2008.04.029]</ref><ref name="Rattanaoudom2012">Rattanaoudom, R., Visvanathan, C., and Boontanon, S.K., 2012. Removal of Concentrated PFOS and PFOA in Synthetic Industrial Wastewater by Powder Activated Carbon and Hydrotalcite. Journal of Water Sustainability, 2(4), pp. 245-248. [http://www.jwsponline.com/uploadpic/Magazine/pp%20245-258.pdf Open access article.] [[Media: Rattanaoudom2012.pdf | Report.pdf]]</ref><ref name="Ziltek2017">Ziltek, 2017. RemBind: Frequently Asked Questions. [https://static1.squarespace.com/static/5c5503db4d546e22f6d2feb2/t/5c733787f9619ae6c84674c9/1551054727451/RemBind+FAQs.pdf Free download] [[Media: RemBind2017.pdf | Report.pdf]]</ref>. Bench-scale tests have shown that activated carbon sorbents reduce leachability of PFAS from soils<ref name="Du2014">Du, Z., Deng, S., Bei, Y., Huang, Q., Wang, B., Huang, J. and Yu, G., 2014. Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents – A review. Journal of Hazardous Materials, 274, pp. 443-454. [https://doi.org/10.1016/j.jhazmat.2014.04.038 DOI: 10.1016/j.jhazmat.2014.04.038]</ref><ref name="Yu2009">Yu, Q., Zhang, R., Deng, S., Huang, J. and Yu, G., 2009. Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: Kinetic and isotherm study. Water Research, 43(4), pp. 1150-1158. [https://doi.org/10.1016/j.watres.2008.12.001 DOI: 10.1016/j.watres.2008.12.001]</ref><ref name="Szabo2017">Szabo, J., Hall, J., Magnuson, M., Panguluri, S., and Meiners, G., 2017. Treatment of Perfluorinated Alkyl Substances in Wash Water Using Granular Activated Carbon and Mixed Media, EPA/600/R-17/175. US Environmental Protection Agency (EPA), Washington, DC. [https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NHSRC&direntryid=337098 Website] [[Media: EPA600R17175.PDF | Report.pdf]]</ref>. A commercial product developed in Australia ([https://rembind.com/ RemBind | + | Various amendments have been manufactured to sorb PFAS to reduce leaching from soil. Although this is a non-destructive approach, stabilization can reduce mass flux from a source area or allow soils to be placed in landfills with reduced potential for leaching. Amendments sorb PFAS through hydrophobic and electrostatic interactions and are applied to soil through ''in situ'' soil mixing or ''ex situ'' stabilization. Effectiveness of amendments varies depending on site conditions, PFAS types present, and mixing conditions<ref name="ITRCwNs2020"/>. Good results have been observed in bench and field scale tests with a variety of cationic clays (natural or chemically modified) and zeolites<ref name="OchoaHerrera2008">Ochoa-Herrera, V., and Sierra-Alvarez, R., 2008. Removal of perfluorinated surfactants by sorption onto granular activated carbon, zeolites and sludge. Chemosphere, 72(10), pp. 1588-1593. [https://doi.org/10.1016/j.chemosphere.2008.04.029 DOI: 10.1016/j.chemosphere.2008.04.029]</ref><ref name="Rattanaoudom2012">Rattanaoudom, R., Visvanathan, C., and Boontanon, S.K., 2012. Removal of Concentrated PFOS and PFOA in Synthetic Industrial Wastewater by Powder Activated Carbon and Hydrotalcite. Journal of Water Sustainability, 2(4), pp. 245-248. [http://www.jwsponline.com/uploadpic/Magazine/pp%20245-258.pdf Open access article.] [[Media: Rattanaoudom2012.pdf | Report.pdf]]</ref><ref name="Ziltek2017">Ziltek, 2017. RemBind: Frequently Asked Questions. [https://static1.squarespace.com/static/5c5503db4d546e22f6d2feb2/t/5c733787f9619ae6c84674c9/1551054727451/RemBind+FAQs.pdf Free download] [[Media: RemBind2017.pdf | Report.pdf]]</ref>. Bench-scale tests have shown that activated carbon sorbents reduce leachability of PFAS from soils<ref name="Du2014">Du, Z., Deng, S., Bei, Y., Huang, Q., Wang, B., Huang, J. and Yu, G., 2014. Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents – A review. Journal of Hazardous Materials, 274, pp. 443-454. [https://doi.org/10.1016/j.jhazmat.2014.04.038 DOI: 10.1016/j.jhazmat.2014.04.038]</ref><ref name="Yu2009">Yu, Q., Zhang, R., Deng, S., Huang, J. and Yu, G., 2009. Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: Kinetic and isotherm study. Water Research, 43(4), pp. 1150-1158. [https://doi.org/10.1016/j.watres.2008.12.001 DOI: 10.1016/j.watres.2008.12.001]</ref><ref name="Szabo2017">Szabo, J., Hall, J., Magnuson, M., Panguluri, S., and Meiners, G., 2017. Treatment of Perfluorinated Alkyl Substances in Wash Water Using Granular Activated Carbon and Mixed Media, EPA/600/R-17/175. US Environmental Protection Agency (EPA), Washington, DC. [https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NHSRC&direntryid=337098 Website] [[Media: EPA600R17175.PDF | Report.pdf]]</ref>. A commercial product developed in Australia ([https://rembind.com/ RemBind™]) combines the cation exchange binding capability of clays, the hydrophobic sorption and [[Wikipedia: Van der Waals force | van der Waals]] attraction of organic material, and the electrostatic interactions of aluminum hydroxide to create a highly effective soil stabilizer. This material has been mixed into soil at 1 to 5% ratio by weight in ''ex situ'' applications and been demonstrated to reduce leachability by greater than 99 percent<ref name="Nolan2015">Nolan, A., Anderson, P., McKay, D., Cartwright, L., and McLean, C., 2015. Treatment of PFCs in Soils, Sediments and Water, WC35. Program and Proceedings, CleanUp Conference 2015. Cooperative Research Centre for Contamination Assessment and Remediation of the Environment (CRC Care), Melbourne, Australia. pp. 374-375. [https://www.crccare.com/files/dmfile/CLEANUP_2015_PROCEEDINGS-web.pdf Free download] [[Media: CRCCare2015.pdf | Report.pdf]]</ref>. |
| − | + | ||
===Thermal Treatment=== | ===Thermal Treatment=== | ||
''Incineration:'' Incineration is a well-developed technology for organics destruction, including PFAS-impacted soils. Incineration is generally defined as high temperature (>1,100°C) thermal destruction of waste, and PFAS are thought to mineralize at high temperatures. Generally, incinerators treat off-gasses by thermal oxidation with temperatures as high as 1,400°C, and vaporized combustion products can be captured using condensation and wet scrubbing<ref name="ITRCwNs2020"/>. Some regulatory officials have expressed concern about possible PFAS emissions in off-gas from these incinerators, and the authors are not aware of any published evidence demonstrating complete mineralization of multiple PFAS in incinerators at the time of this posting. In general, incineration is designed to provide “5 nines of destruction” – destruction of 99.999% of the contaminants, although incinerators are not designed to specifically treat PFAS to this standard. In the absence of approved industry standard test methods, the US EPA is developing off-gas/stack testing procedures capable of detecting PFAS at the levels considered to be harmful<ref name="EPA2018">US Environmental Protection Agency (EPA), 2018. PFAS Research and Development, Community Engagement in Fayetteville, North Carolina. [https://www.epa.gov/pfas/pfas-community-engagement-north-carolina-meeting-materials Website] [[Media: EPAFayetteville2018.pdf | Report.pdf]]</ref>. | ''Incineration:'' Incineration is a well-developed technology for organics destruction, including PFAS-impacted soils. Incineration is generally defined as high temperature (>1,100°C) thermal destruction of waste, and PFAS are thought to mineralize at high temperatures. Generally, incinerators treat off-gasses by thermal oxidation with temperatures as high as 1,400°C, and vaporized combustion products can be captured using condensation and wet scrubbing<ref name="ITRCwNs2020"/>. Some regulatory officials have expressed concern about possible PFAS emissions in off-gas from these incinerators, and the authors are not aware of any published evidence demonstrating complete mineralization of multiple PFAS in incinerators at the time of this posting. In general, incineration is designed to provide “5 nines of destruction” – destruction of 99.999% of the contaminants, although incinerators are not designed to specifically treat PFAS to this standard. In the absence of approved industry standard test methods, the US EPA is developing off-gas/stack testing procedures capable of detecting PFAS at the levels considered to be harmful<ref name="EPA2018">US Environmental Protection Agency (EPA), 2018. PFAS Research and Development, Community Engagement in Fayetteville, North Carolina. [https://www.epa.gov/pfas/pfas-community-engagement-north-carolina-meeting-materials Website] [[Media: EPAFayetteville2018.pdf | Report.pdf]]</ref>. | ||
Revision as of 14:18, 5 February 2021
PFAS Soil Remediation Technologies
Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) are mobile in the subsurface and highly resistant to natural degradation processes, therefore soil source areas can be ongoing sources of groundwater contamination. The United States Environmental Protection Agency (US EPA) has not promulgated soil standards for any PFAS, although a handful of states have for select compounds. Soil standards issued for protection of groundwater are in the single digit part per billion range, which is a very low threshold for soil impacts. Well developed soil treatment technologies are limited to capping, excavation with incineration or disposal, and soil stabilization with sorptive amendments. At present, no in situ destructive soil treatment technologies have been demonstrated.
Related Article(s):
Contributor(s): Jim Hatton and Bill DiGuiseppi
Key Resource(s):
- ITRC Fact Sheet: Treatment Technologies, PFAS – Per- and Polyfluoroalkyl Substances[1].
- Persistence of Perfluoroalkyl Acid Precursors in AFFF-Impacted Groundwater and Soil[2].
Introduction
PFAS are a class of highly fluorinated compounds including perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA), and many other compounds with a variety of industrial and consumer uses. These compounds are often highly resistant to treatment[3] and the more mobile compounds are often problematic in groundwater systems[4]. The US EPA has published lifetime drinking water health advisories for the combined concentration of 70 nanograms per liter (ng/L) for two common and recalcitrant PFAS: PFOS, a perfluoroalkyl sulfonic acid (PFSA), and PFOA, a perfluoroalkyl carboxylic acid (PFCA)[5][6].(See Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) for nomenclature.)
While many of the earliest sites where these compounds were detected in groundwater were manufacturing sites, some recent detections have been attributed to fire training activities associated with aqueous film-forming foams (AFFF). AFFF is the US Department of Defense (DoD) designation for Class B firefighting foam containing PFAS, which is required for fighting fires involving petroleum liquids. Fire training areas and other source areas where AFFF was released at the surface have the potential to be ongoing sources of groundwater contamination[2]. (See also PFAS Sources.)
No national soil cleanup standards have been promulgated by the US EPA, although Regional Screening Levels (RSLs) have been calculated and published for perfluorobutane sulfonate (PFBS)[7] and data are available to calculate RSLs for PFOA and PFOS[8]. Several states have promulgated standards[9] or screening levels[10][11][12][13][14] for soil concentrations protective of groundwater, which are several orders of magnitude lower than direct dermal exposure guidelines. These single-digit part per billion criteria will likely drive remedial actions in PFAS source areas in the future. At present, the lack of federally promulgated standards and uncertainty about future standards causes temporary stockpiling of PFAS-impacted soils on sites with soil generated from construction or investigation activities.
Soil Treatment
Addressing recalcitrant contaminants in soil has traditionally been done through containment/capping or excavation and off-site disposal or treatment. Containment/capping may be an acceptable solution for PFAS in some locations. However, containment/capping is not considered ideal given the history of releases from engineered landfills and restrictions on use of land containing capped soils. Innovative treatment approaches for PFAS include stabilization with amendments and thermal treatment.
Excavation and Disposal
Excavation and off-site disposal or treatment of PFAS-impacted soils is the only well-developed treatment technology option and may be acceptable for small quantities of soil, such as those generated during characterization activities (i.e., investigation derived waste, IDW). Disposal in non-hazardous landfills is allowable in most states. However, some landfill operators are choosing to restrict acceptance of PFAS-containing waste and soils as a protection against future liability. In addition, the US EPA and some states are considering or have designated PFOA and PFOS as hazardous substances, which would reduce the number of facilities where disposal of PFAS-contaminated soil would be allowed[15]. Treatment of excavated soils is commonly performed using incineration or other high temperature thermal methods[1]. Recent negative publicity regarding incomplete combustion of PFAS in incinerators[16] has caused some states to ban PFAS incineration[17].
Stabilization
Various amendments have been manufactured to sorb PFAS to reduce leaching from soil. Although this is a non-destructive approach, stabilization can reduce mass flux from a source area or allow soils to be placed in landfills with reduced potential for leaching. Amendments sorb PFAS through hydrophobic and electrostatic interactions and are applied to soil through in situ soil mixing or ex situ stabilization. Effectiveness of amendments varies depending on site conditions, PFAS types present, and mixing conditions[8]. Good results have been observed in bench and field scale tests with a variety of cationic clays (natural or chemically modified) and zeolites[18][19][20]. Bench-scale tests have shown that activated carbon sorbents reduce leachability of PFAS from soils[21][22][23]. A commercial product developed in Australia (RemBind™) combines the cation exchange binding capability of clays, the hydrophobic sorption and van der Waals attraction of organic material, and the electrostatic interactions of aluminum hydroxide to create a highly effective soil stabilizer. This material has been mixed into soil at 1 to 5% ratio by weight in ex situ applications and been demonstrated to reduce leachability by greater than 99 percent[24].
Thermal Treatment
Incineration: Incineration is a well-developed technology for organics destruction, including PFAS-impacted soils. Incineration is generally defined as high temperature (>1,100°C) thermal destruction of waste, and PFAS are thought to mineralize at high temperatures. Generally, incinerators treat off-gasses by thermal oxidation with temperatures as high as 1,400°C, and vaporized combustion products can be captured using condensation and wet scrubbing[8]. Some regulatory officials have expressed concern about possible PFAS emissions in off-gas from these incinerators, and the authors are not aware of any published evidence demonstrating complete mineralization of multiple PFAS in incinerators at the time of this posting. In general, incineration is designed to provide “5 nines of destruction” – destruction of 99.999% of the contaminants, although incinerators are not designed to specifically treat PFAS to this standard. In the absence of approved industry standard test methods, the US EPA is developing off-gas/stack testing procedures capable of detecting PFAS at the levels considered to be harmful[25].
Thermal Desorption: Thermal Desorption of PFAS from soil has been demonstrated at the field scale in Australia and the US (Alaska)[24] using a rotary kiln operating at temperatures in the range of 900°C or less with treatment times of 10-15 minutes[26]. At these temperatures, some PFAS are mineralized, releasing fluorine that must be captured in off-gas treatment systems. Some PFAS would not be destroyed at these temperatures and therefore must be captured in off-gas treatment systems. Several bench-scale tests have been performed that have narrowed down the optimal temperature for desorption to between 350°C and 400°C[27][28]. A US Department of Defense (DoD) Strategic Environmental Research and Development Program (SERDP) field-scale demonstration was performed in Oregon, where thermal desorption was conducted at 400°C over several days, and the PFAS were captured on vapor-phase activated carbon and incinerated[27]. An 'in situ' thermal desorption project has been funded under the US DoD’s Environmental Security Technology Certification Program (ESTCP) to demonstrate that vadose zone soil can be heated to the requisite 350°C and held there for the appropriate length of time to desorb and capture PFAS from soil source areas[29].
Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) are a complex family of more than 3,000 manmade fluorinated organic chemicals[30] although not all of these are currently in use or production. PFAS are produced using several different processes. Fluorosurfactants, which include perfluoroalkyl acids (PFAAs) (see PFAS article for nomenclature) and side-chain fluorinated polymers, have been manufactured using two major processes: electrochemical fluorination (ECF) and telomerization[31]. ECF was licensed by 3M in the 1940s[32] and used by 3M until 2001. ECF produces a mixture of even and odd numbered carbon chain lengths of approximately 70% linear and 30% branched substances[33]. Telomerization was developed in the 1970s[34], and yields mainly even numbered, straight carbon chain isomers[3][35]. PFAS manufacturers have provided PFAS to secondary manufacturers for production of a vast array of industrial and consumer products.
During manufacturing, PFAS may be released into the atmosphere then redeposited on land where they can also affect surface water and groundwater, or PFAS may be discharged without treatment to wastewater treatment plants or landfills, and eventually be released into the environment by treatment systems that are not designed to mitigate PFAS (see also PFAS Transport and Fate). Industrial discharges of PFAS were unregulated for many years, but that has begun to change. In January 2016, New York became the first state in the nation to regulate PFOA as a hazardous substance followed by the regulation of PFOS in April 2016. Consumer and industrial uses of PFAS-containing products can also end up releasing PFAS into landfills and into municipal wastewater, where it may accumulate undetected in biosolids which are typically treated by land application.
Industrial Sources
PFAS are used in many industrial and consumer applications, which may have released PFAS into the environment and impacted drinking water supplies in many areas of the United States[36]. Both in the United States (US) and abroad, primary manufacturing facilities produce PFAS and secondary manufacturing facilities use PFAS to produce goods. Environmental release mechanisms associated with these facilities include air emission and dispersion, spills, and disposal of manufacturing wastes and wastewater. Potential impacts to air, soil, sediment, surface water, stormwater, and groundwater are present not only at primary release points but potentially over the surrounding area[37]. Some of the potential primary and secondary sources of PFAS releases to the environment are listed here[1]:
- Textiles and leather: Factory or consumer applied coating to repel water, oil, and stains. Applications include protective clothing and outerwear, umbrellas, tents, sails, architectural materials, carpets, and upholstery[38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53].
- Paper products: Surface coatings to repel grease and moisture. Uses include non-food paper packaging (for example, cardboard, carbonless forms, masking papers) and food-contact materials (for example, pizza boxes, fast food wrappers, microwave popcorn bags, baking papers, pet food bags)[38][3][39][41][44][48][49][52][54]
- Metal Plating & Etching: Corrosion prevention, mechanical wear reduction, aesthetic enhancement, surfactant, wetting agent/fume suppressant for chrome, copper, nickel and tin electroplating, and post-plating cleaner[55][56][3][42][57][49][58][31][59]
- Industrial Surfactants, Resins, Molds, Plastics: Manufacture of plastics and fluoropolymers, rubber, and compression mold release coatings; plumbing fluxing agents; fluoroplastic coatings, composite resins, and flame retardant for polycarbonate[3][62][41][63][42][64][60][48][50][52][65]
- Photolithography, Semiconductor Industry: Photoresists, top anti-reflective coatings, bottom anti-reflective coatings, and etchants, with other uses including surfactants, wetting agents, and photo-acid generation[66][67][40][60][49][50]
Class B Firefighting Foams
Aqueous film forming foam (AFFF) and other fluorinated Class B firefighting foams are another important source of PFAS to the environment, especially in military and aviation settings. Class B firefighting foams have been used since the 1960s to extinguish flammable liquid hydrocarbon fires and for vapor suppression. These foams contain complex and variable mixtures of PFAS that act as surfactants. Fluorinated surfactants are both hydrophobic and oleophobic (oil-repelling), as well as thermally stable, chemically stable, and highly surface active[68]. These properties make them uniquely suited to fighting hydrocarbon fuel fires. Use of fluorinated Class B foams is prevalent and is a major source of PFAS to the environment. Release to the environment typically occurs during firefighting operations, firefighter training, apparatus testing, or leakage during storage. Research into fluorine-free alternatives is underway and Congressional pressure is leading towards banning fluorinated Class B firefighting foams in the United States.
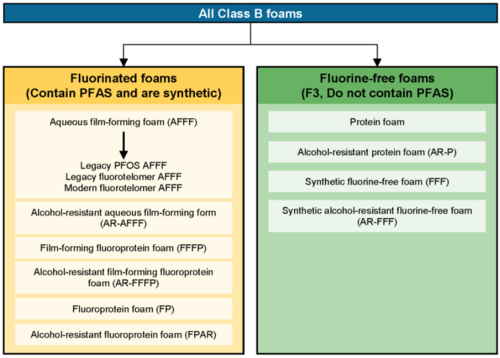
When discussing the relationship between firefighting foams and sources of PFAS to the environment, the emphasis is typically on AFFF; however, many different types of Class B firefighting foams exist. These may or may not be fluorinated (contain PFAS). Class B foams are used to extinguish Class B fires, that is, those involving flammable liquids. Fluorinated Class B foams spread across the surface of the flammable liquid forming a thin film and extinguish fires by (1) excluding air from the flammable vapors, (2) suppressing vapor release, (3) physically separating the flames from the fuel source, and (4) cooling the fuel surface and surrounding metal surfaces[69]. From a PFAS perspective, Class B firefighting foams can be divided into two broad categories: fluorinated foams (that contain PFAS) and fluorine-free foams (that do not contain PFAS)[1]. This distinction and examples of each type are shown in Figure 1.
AFFF was developed by the US Navy in the 1960s and in 1969, the US Department of Defense (DoD) issued military specification MIL-F-24385 listing firefighting performance requirements for all AFFF used within the US DoD[1][70][71]. These performance standards are often referred to as “Mil-Spec.” Products that meet the Mil-Spec have been added to the US DoD Qualified Product Listing (QPL). In 2006 the US Federal Aviation Administration (FAA) also began requiring that 14-CFR-139-certified commercial airports purchase Mil-Spec compliant AFFF only. Because the US DoD and FAA have been the primary purchasers of AFFF, development of AFFF product mixtures has historically been performance-driven (to comply with the Mil-Spec) rather than formula-driven (the specific PFAS mixtures utilized have varied over time and by manufacturer). Multiple manufacturers in the US and throughout the world produce or have produced AFFF concentrate[1]. AFFF concentrate is or has been available in 1%, 3%, or 6% formulations, where the percentage designates the recommended percentage of concentrate to be mixed into water during application.
The specific mixtures of PFAS found in AFFF have varied by manufacturer and over time due to differences in production processes and voluntary formula changes. AFFF formulations can generally be grouped into three categories[1]:
- Legacy Perfluorooctane Sulfonate (PFOS) AFFF This type of AFFF was manufactured exclusively by 3M under the brand name “Lightwater” from the late 1960s until 2002 using the ECF production process. They contain PFOS and perflouroalkane sulfonates (PFSAs) such as perfluorohexane sulfonate (PFHxS)[1][4]. Legacy PFOS AFFF produced by ECF were voluntarily phased out in 2002, however, use of stockpiled product was permitted after that date[1].
- Legacy fluorotelomer AFFF This group consists of AFFF manufactured and sold in the U.S. from the 1970s until 2016 and includes all brands that were produced using a process known as fluorotelomerization (FT). The FT manufacturing process produces polyfluorinated substances that can degrade in the environment to perfluoroalkyl substances (specifically PFAAs) including Perfluorooctanoic Acid (PFOA). Polyfluoroalkyl substances that degrade to create terminal PFAAs are referred to as “precursors” [1].
- Modern fluorotelomer AFFF This group consists of AFFF developed in response to the USEPA 2010-2015 voluntary PFOA Stewardship Program[72], which asked companies to commit to first reducing and then eliminating the following: PFOA, precursors that can break down to PFOA, and related chemicals from facility emissions and products. In response, manufacturers began producing only short-chain fluorosurfactants targeting fluorotelomer PFAS with 6 carbons per chain (C6), rather than the traditional long-chain fluorosurfactants (8 or more carbons per chain). These short-chain PFAS do not breakdown in the environment to PFOS or PFOA[1]. Their toxicity in comparison to long-chain fluorosurfactants is a topic of current research.
In the US, AFFF users including the US DoD (predominantly the Navy and Air Force), civilian airports, oil refineries, other petrochemical industries, and municipal fire departments[73]. AFFF is used, for example, in fire fighting vehicles, in fixed fire suppression systems (including sprinklers and fixed spray systems in or at aircraft hangars, flammable liquid storage areas, engine hush houses, and fuel farms), and onboard military and commercial ships. Fluorinated Class B foams may be introduced to the environment through the following practices[1]:
- low volume releases of foam concentrate during storage, transfer or operational requirements that mandate periodic equipment calibration
- moderate volume discharge of foam solution for apparatus testing and episodic discharge of AFFF-containing fire suppression systems within large aircraft hangars and buildings
- occasional, high-volume, broadcast discharge of foam solution for firefighting and fire suppression/prevention for emergency response
- periodic, high volume, broadcast discharge for fire training
- accidental leaks from foam distribution piping between storage and pumping locations, and from storage tanks and railcars
The DoD is currently replacing legacy, long-chain AFFF with modern, short-chain fluorotelomer AFFF and disposing of the legacy foams through incineration. While the PFAS included in modern fluorotelomer AFFF formulations are currently understood to be less toxic and less bioaccumulative than those used in legacy formulations, they are also environmentally persistent and can degrade to produce other PFAS that may pose environmental concerns[1]. While fluorine free alternatives exist, they do not meet the current Mil-Spec[71] which requires that fluorine-based compounds be used. The US DoD is working to revise the Mil-Spec to allow fluorine-free foams, and several states have passed laws prohibiting the use of fluorinated Class B foams for training and prohibiting future manufacture, sale or distribution of fluorinated foams, with limited exceptions[74] (e.g., WA Rev Code § 70.75A.005 (2019); VA § 9.1-207.1 (2019)). Additionally, a bill passed in the US Congress in 2018 directs the FAA to allow fluorine-free foams for use at commercial airports[75]. Research into the development of Mil-Spec compliant fluorine-free foams that will be compatible with existing AFFF and supporting equipment is ongoing and includes the following:
- Novel Fluorine-Free Replacement for Aqueous Film Forming Foam (Lead investigator: Dr. Joseph Tsang, Naval Air Warfare Center Weapons Divisions) SERDP/ESTCP Project WP-2737
- Fluorine-Free Aqueous Film Forming Foam (Lead investigator: Dr. John Payne, National Foam) SERDP/ESTCP Project WP-2738
- Fluorine-Free Foams with Oleophobic Surfactants and Additives for Effective Pool fire Suppression (Lead investigator: Dr. Ramagopal Ananth, U.S. Naval Research Laboratory) SERDP/ESTCP Project WP-2739
Wastewater Treatment Plants
Consumer and/or industrial uses of PFAS-containing materials results in the discharge of PFAS to industrial and municipal wastewater treatment plants (WWTPs). Conventional WWTP treatment processes remove less than 5% of PFAAs[47][76][77]. WWTPs, particularly those that receive industrial wastewater, are possible sources of PFAS release[78][79][80].
Evaluation of full-scale WWTPs has indicated that conventional primary (sedimentation and clarification) and secondary (aerobic biodegradation of organic matter) treatment processes can result in changes in PFAS concentrations and classes. For example, higher concentrations of PFAAs have been observed in effluent than in influent, presumably due to transformation of precursor PFAS[76]. Some data has indicated that the terminal PFAS compounds PFOS and PFOA were among the most frequently detected PFAS in wastewater[81]. A state-wide study in Michigan indicated that PFAS were detected in all of the samples from 42 WWTPs, including influent, effluent, and biosolids/sludge samples, and that the short-chain PFAS were more frequently detected in the liquid process flow (influent and effluent), while long-chain PFAS were more common in biosolids[11].
Multiple studies have found PFAS in municipal sewage sludge[82][11]. The US EPA states that more than half of the sludge produced in the United States is applied to agricultural land as biosolids, therefore there are concerns that biosolids applications may become a potential source of PFAS to the environment[83]. Application of biosolids as a soil amendment can potentially result in transfer of PFAS to soil, surface water and groundwater and can possibly allow PFAS to enter the food chain[84][85][86][87][88]. Limited studies have shown that PFAS concentrations can be elevated in surface and groundwater in the vicinity of agricultural fields that received PFAS contaminated biosolids for an extended period[89]. The most abundant PFAS found in biosolids are the long-chain PFAS[81][11]. Based on the persistence and stability of long-chain PFAS and their interaction with biosolids, research is ongoing to determine PFAS leachability from biosolids and their bioavailability for uptake by plants, soil organisms, and the consumers of potentially PFAS-impacted plants and soil organisms.
Solid Waste Management Facilities
Industrial, commercial, and consumer products containing PFAS that have been disposed in municipal solid waste (MSW) landfills or other legacy disposal areas since the 1950s are potential sources of PFAS release to the environment. Environmental and drinking water impacts from disposal of legacy PFAS-containing industrial and consumer wastes have been documented[90][37][91].
Several studies have identified a wide variety of PFAS in MSW landfill leachates[92][93]. PFAS composition and concentration in leachates vary depending on waste age, climate, and waste composition[94][95]. The relative concentrations of various PFAS in leachate and groundwater from landfill sites is different from those found at WWTPs and AFFF-contaminated sites. In particular, 5:3 fluorotelomer carboxylic acid (FTCA) is a common and often dominant PFAS found in landfills, and has been released from carpet in model anaerobic landfill reactors. This compound could prove to be an indicator that PFAS in the environment originated from a landfill[96][95]. PFAS may also be released to the air from landfills, predominantly as fluorotelomer alcohols (FTOHs) and perfluorobutanoate (PFBA). In one study, total airborne PFAS concentrations were 5 to 30 times greater at landfills than at background reference sites[97]. PFAS release rates within landfills vary over time for a given waste mass, with climate (for example, rainfall) serving as the apparent driving factor for the variations[95][98].
Commercial and Consumer Products
PFAS are widely used in consumer products and household applications, with a diverse mixture of PFAS found in varying concentrations depending on the product[99][100][101][102][53][52][31][103]. Environmental releases associated with the commercial and consumer products are primarily related to their production. To a much lower extent, the environmental releases may be associated with the management of solid waste (for example, disposal of used items in a MSW landfill) and wastewater disposal (for example, discharge to WWTPs, private septic systems, or other subsurface disposal systems).
Studies have shown that physical degradation of some consumer products (such as PFAS-treated paper, textiles, and carpets) may release PFAS in house dust[104]. Additionally, studies have also shown that professional ski wax technicians may have significant inhalation exposures to PFAS[105] and snowmelt and surface waters near ski areas could have measurable PFAS impacts[106].
As increased environmental sampling for PFAS occurs, additional information will become available to further our understanding of the major and minor PFAS contributors to the environment.
References
- ^ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 Interstate Technology and Regulatory Council (ITRC), 2020. PFAS Technical and Regulatory Guidance Document and Fact Sheets, PFAS-1. PFAS Team, Washington, DC. Website Report.pdf
- ^ 2.0 2.1 Houtz, E.F., Higgins, C.P., Field, J.A., and Sedlak, D.L., 2013. Persistence of Perfluoroalkyl Acid Precursors in AFFF-Impacted Groundwater and Soil. Environmental Science and Technology, 47(15), pp. 8187−8195. DOI: 10.1021/es4018877
- ^ 3.0 3.1 3.2 3.3 3.4 3.5 Kissa, Erik, 2001. Fluorinated Surfactants and Repellents: Second Edition. Surfactant Science Series, Volume 97. Marcel Dekker, Inc., CRC Press, New York. 640 pages. ISBN 978-0824704728
- ^ 4.0 4.1 Backe, W.J., Day, T.C., and Field, J.A., 2013. Zwitterionic, Cationic, and Anionic Fluorinated Chemicals in Aqueous Film Forming Foam Formulations and Groundwater from U.S. Military Bases by Nonaqueous Large-Volume Injection HPLC-MS/MS. Environmental Science and Technology, 47(10), pp. 5226-5234. DOI: 10.1021/es3034999
- ^ US Environmental Protection Agency (EPA), 2016. Drinking Water Health Advisory for Perfluorooctane Sulfonate (PFOS), EPA 822-R-16-004. Office of Water, Health and Ecological Criteria Division, Washington, DC. Free download from US EPA Report.pdf
- ^ US Environmental Protection Agency (EPA), 2016. Drinking Water Health Advisory for Perfluorooctanoic Acid (PFOA), EPA 822-R-16-005. Office of Water, Health and Ecological Criteria Division, Washington, DC. Free download from US EPA Report
- ^ US Environmental Protection Agency (EPA), 2020. Regional Screening Levels (RSLs) – User's Guide. Washington, DC. Website
- ^ 8.0 8.1 8.2 Interstate Technology Regulatory Council (ITRC), 2020. PFAS Water and Soil Values Table. PFAS – Per- and Polyfluoroalkyl Substances: PFAS Fact Sheets. Free download. 2020 Water and Soil Tables (excel file)
- ^ Alaska Department of Environmental Conservation (AK DEC), 2020. 18 AAC 75, Oil and Other Hazardous Substances Pollution Control. Anchorage, AK. Free download. Report.pdf
- ^ Maine Department of Environmental Protection (ME DEP), 2018. Maine Remedial Action Guidelines (RAGs) for Sites Contaminated with Hazardous Substances. Augusta, ME. Free download. Report.pdf
- ^ 11.0 11.1 11.2 11.3 Michigan Department of Environment, Great Lakes, and Energy (EGLE), 2020. Cleanup Criteria Requirements for Response Activity (Formerly the Part 201 Generic Cleanup Criteria and Screening Levels). Remediation and Redevelopment Division, Lansing, MI. Website
- ^ Nebraska Department of Energy and Environment (NE DEE), 2018. Voluntary Cleanup Program Remedial Goals, Table A-1: Groundwater and Soil Remediation Goals. Lincoln, NE. Free download. Report.pdf
- ^ North Carolina Department of Environmental Quality (NC DEQ), 2020. Preliminary Soil Remediation Goals (PSRG) Table. Raleigh, NC. Free download. Report.pdf
- ^ Texas Commission on Environmental Quality (TCEQ), 2021. Texas Risk Reduction Program (TRRP), Tier 1 Protective Concentration Levels (PCL) Tables. Free Download. 2021 PCL Tables (excel file)
- ^ US Environmental Protection Agency (EPA), 2019. EPA’s Per- and Polyfluoroalkyl Substances (PFAS) Action Plan: EPA 823R18004. Washington, DC. Website Report.pdf 2020 Update
- ^ Cheryl Hogue, 2020. Incineration may spread, not break down PFAS. Chemical and Engineering News, American Chemical Society. Website Report.pdf
- ^ New York State Senate, 2020. An ACT prohibiting the incineration of aqueous film-forming foam containing perfluoroalkyl and polyfluoroalkyl substances in certain cities. Website Report.pdf
- ^ Ochoa-Herrera, V., and Sierra-Alvarez, R., 2008. Removal of perfluorinated surfactants by sorption onto granular activated carbon, zeolites and sludge. Chemosphere, 72(10), pp. 1588-1593. DOI: 10.1016/j.chemosphere.2008.04.029
- ^ Rattanaoudom, R., Visvanathan, C., and Boontanon, S.K., 2012. Removal of Concentrated PFOS and PFOA in Synthetic Industrial Wastewater by Powder Activated Carbon and Hydrotalcite. Journal of Water Sustainability, 2(4), pp. 245-248. Open access article. Report.pdf
- ^ Ziltek, 2017. RemBind: Frequently Asked Questions. Free download Report.pdf
- ^ Du, Z., Deng, S., Bei, Y., Huang, Q., Wang, B., Huang, J. and Yu, G., 2014. Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents – A review. Journal of Hazardous Materials, 274, pp. 443-454. DOI: 10.1016/j.jhazmat.2014.04.038
- ^ Yu, Q., Zhang, R., Deng, S., Huang, J. and Yu, G., 2009. Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: Kinetic and isotherm study. Water Research, 43(4), pp. 1150-1158. DOI: 10.1016/j.watres.2008.12.001
- ^ Szabo, J., Hall, J., Magnuson, M., Panguluri, S., and Meiners, G., 2017. Treatment of Perfluorinated Alkyl Substances in Wash Water Using Granular Activated Carbon and Mixed Media, EPA/600/R-17/175. US Environmental Protection Agency (EPA), Washington, DC. Website Report.pdf
- ^ 24.0 24.1 Nolan, A., Anderson, P., McKay, D., Cartwright, L., and McLean, C., 2015. Treatment of PFCs in Soils, Sediments and Water, WC35. Program and Proceedings, CleanUp Conference 2015. Cooperative Research Centre for Contamination Assessment and Remediation of the Environment (CRC Care), Melbourne, Australia. pp. 374-375. Free download Report.pdf
- ^ US Environmental Protection Agency (EPA), 2018. PFAS Research and Development, Community Engagement in Fayetteville, North Carolina. Website Report.pdf
- ^ Burke, Jill, 2019. Fairbanks incinerator shows promise for cleaning toxic soil. Channel 2-KTUU, October 8. Website
- ^ 27.0 27.1 Hatton, J., Dasu, K., Richter, R., Fitzpatrick, T., and Higgins, C., 2019. Field Demonstration of Infrared Thermal Treatment of PFAS-impacted Soils from Subsurface Investigations. Strategic Environmental Research and Development Program (SERDP), Project ER18-1603, Alexandria, VA. Website Report.pdf
- ^ DiGuiseppi, W., Richter, R., and Riggle, M., 2019. Low Temperature Desorption of Per- and Polyfluoroalkyl Substances. The Military Engineer, 111(719), pp. 52-53. Society of American Military Engineers, Washington, DC. Open access article. Report.pdf
- ^ Iery, R., 2020. In Situ Thermal Treatment of PFAS in the Vadose Zone. US Department of Defense, Environmental Security Technology Certification Program (ESTCP), Project ER20-5250. Website
- ^ Wang, Z., DeWitt, J.C., Higgins, C.P., and Cousins, I.T., 2017. A Never-Ending Story of Per- and Poly-Fluoroalkyl Substances (PFASs)? Environmental Science and Technology, 51(5), pp. 2508-2518. DOI: 10.1021/acs.est.6b04806 Open access article.
- ^ 31.0 31.1 31.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedKEMI2015 - ^ Banks, R.E., Smart, B.E. and Tatlow, J.C. eds., 1994. Organofluorine Chemistry: Principles and Commercial Applications. Springer Science and Business Media, New York, N. Y. DOI: 10.1007/978-1-4899-1202-2
- ^ Concawe (Conservation of Clean Air and Water in Europe), 2016. Environmental fate and effects of poly- and perfluoroalkyl substances (PFAS). Report No. 8/16. Brussels, Belgium. Report.pdf
- ^ Benskin, J.P., Ahrens, L., Muir, D.C., Scott, B.F., Spencer, C., Rosenberg, B., Tomy, G., Kylin, H., Lohmann, R. and Martin, J.W., 2012. Manufacturing Origin of Perfluorooctanoate (PFOA) in Atlantic and Canadian Arctic Seawater. Environmental Science and Technology, 46(2), pp. 677-685. DOI: 10.1021/es202958p
- ^ Parsons, J.R., Sáez, M., Dolfing, J. and De Voogt, P., 2008. Biodegradation of Perfluorinated Compounds. Reviews of Environmental Contamination and Toxicology, 196, pp. 53-71. Springer, New York, NY. DOI: 10.1007/978-0-387-78444-1_2 Free download from: ResearchGate
- ^ Environmental Working Group (EWG) and Northeastern University Social Science Environmental Health Research Institute, 2017. Mapping A Contamination Crisis. Website
- ^ 37.0 37.1 Shin, H.M., Vieira, V.M., Ryan, P.B., Detwiler, R., Sanders, B., Steenland, K., and Bartell, S.M., 2011. Environmental Fate and Transport Modeling for Perfluorooctanoic Acid Emitted from the Washington Works Facility in West Virginia. Environmental Science and Technology, 45(4), pp. 1435-1442. DOI: 10.1021/es102769t
- ^ 38.0 38.1 Rao, N.S., and Baker, B.E., 1994. Textile Finishes and Fluorosurfactants. In: Organofluorine Chemistry, Banks, R.E., Smart, B.E., and Tatlow, J.C., Eds. Springer, New York. DOI: 10.1007/978-1-4899-1202-2_15
- ^ 39.0 39.1 Hekster, F.M., Laane, R.W. and De Voogt, P., 2003. Environmental and Toxicity Effects of Perfluoroalkylated Substances. Reviews of Environmental Contamination and Toxicology, 179, pp. 99-121. Springer, New York, NY. DOI: 10.1007/0-387-21731-2_4
- ^ 40.0 40.1 Brooke, D., Footitt, A., and Nwaogu, T.A., 2004. Environmental Risk Evaluation Report: Perfluorooctanesulphonate (PFOS). Environment Agency (UK), Science Group. Free download from: The Stockholm Convention Report.pdf
- ^ 41.0 41.1 41.2 Poulsen, P.B., Jensen, A.A., and Wallström, E., 2005. More environmentally friendly alternatives to PFOS-compounds and PFOA. Danish Environmental Protection Agency, Environmental Project 1013. Report.pdf
- ^ 42.0 42.1 42.2 Prevedouros, K., Cousins, I.T., Buck, R.C. and Korzeniowski, S.H., 2006. Sources, Fate and Transport of Perfluorocarboxylates. Environmental Science and Technology, 40(1), pp. 32-44. DOI: 10.1021/es0512475 Free download from: Academia.edu
- ^ Walters, A., and Santillo, D., 2006. Technical Note 06/2006: Uses of Perfluorinated Substances. Greenpeace Research Laboratories. Website Report.pdf
- ^ 44.0 44.1 Trudel, D., Horowitz, L., Wormuth, M., Scheringer, M., Cousins, I.T. and Hungerbühler, K., 2008. Estimating Consumer Exposure to PFOS and PFOA. Risk Analysis: An International Journal, 28(2), pp. 251-269. DOI: 10.1111/j.1539-6924.2008.01017.x
- ^ Guo, Z., Liu, X., Krebs, K.A. and Roache, N.F., 2009. Perfluorocarboxylic Acid Content in 116 Articles of Commerce, EPA/600/R-09/033. National Risk Management Research Laboratory, US Environmental Protection Agency, Washington, DC. Available from: US EPA. Report.pdf
- ^ US Environmental Protection Agency (USEPA), 2009. Long-Chain Perfluorinated Chemicals (PFCs), Action Plan. Website Report.pdf
- ^ 47.0 47.1 Ahrens, L., 2011. Polyfluoroalkyl compounds in the aquatic environment: a review of their occurrence and fate. Journal of Environmental Monitoring, 13(1), pp.20-31. DOI: 10.1039/C0EM00373E. Free download available from: ResearchGate
- ^ 48.0 48.1 48.2 Buck, R.C., Franklin, J., Berger, U., Conder, J.M., Cousins, I.T., De Voogt, P., Jensen, A.A., Kannan, K., Mabury, S.A. and van Leeuwen, S.P., 2011. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integrated Environmental Assessment and Management, 7(4), pp. 513-541. DOI: 10.1002/ieam.258 Open access article.
- ^ 49.0 49.1 49.2 49.3 United Nations Environmental Programme (UNEP), 2011. Report of the persistent organic pollutants review committee on the work of its sixth meeting, Addendum, Guidance on alternatives to perfluorooctane sulfonic acid and its derivatives, UNEP/POPS/POPRC.6/13/Add.3/Rev.1 Website Report.pdf
- ^ 50.0 50.1 50.2 Herzke, D., Olsson, E. and Posner, S., 2012. Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in consumer products in Norway – A pilot study. Chemosphere, 88(8), pp. 980-987. DOI: 10.1016/j.chemosphere.2012.03.035
- ^ Patagonia, Inc., 2016. An Update on Our DWR Problem. Website Report.pdf
- ^ 52.0 52.1 52.2 52.3 Kotthoff, M., Müller, J., Jürling, H., Schlummer, M., and Fiedler, D., 2015. Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environmental Science and Pollution Research, 22(19), pp. 14546-14559. DOI: 10.1007/s11356-015-4202-7 Open access article.
- ^ 53.0 53.1 Agency for Toxic Substances and Disease Registry (ATSDR), 2018. Toxicological Profile for Perfluoroalkyls, Draft for Public Comment. US Department of Health and Human Services. Free download from: ATSDR Report.pdf
- ^ Schaider, L.A., Balan, S.A., Blum, A., Andrews, D.Q., Strynar, M.J., Dickinson, M.E., Lunderberg, D.M., Lang, J.R., and Peaslee, G.F., 2017. Fluorinated Compounds in US Fast Food Packaging. Environmental Science and Technology Letters, 4(3), pp. 105-111. DOI: 10.1021/acs.estlett.6b00435 Open access article.
- ^ US Environmental Protection Agency (USEPA), 1996. Emission Factor Documentation for AP-42, Section 12.20. Office of Air Quality Planning and Standards, Emission Factor and Inventory Group, Research Triangle Park, NC. Report.pdf
- ^ Riordan, B.J., Karamchandanl, R.T., Zitko, L.J., and Cushnie Jr., G.C., 1998. Capsule Report: Hard Chrome Fume Suppressants and Control Technologies. Center for Environmental Research Information, National Risk Management Research Laboratory, Office of Research and Development. EPA/625/R-98/002 Website Report.pdf
- ^ US Environmental Protection Agency (USEPA), 2009. PFOS Chromium Electroplater Study. US EPA – Region 5, Chicago, IL. Report.pdf
- ^ Occupational Safety and Health Agency (OSHA), 2013. Fact Sheet: Controlling Hexavalent Chromium Exposures during Electroplating. United States Department of Labor. Report.pdf
- ^ Danish Environmental Protection Agency, 2015. Alternatives to perfluoroalkyl and polyfluoroalkyl substances (PFAS) in textiles. Report.pdf
- ^ 60.0 60.1 60.2 van der Putte, I., Murin, M., van Velthoven, M., and Affourtit, F., 2010. Analysis of the risks arising from the industrial use of Perfluorooctanoic acid (PFOA) and Ammonium Perfluorooctanoate (APFO) and from their use in consumer articles. Evaluation of the risk reduction measures for potential restrictions on the manufacture, placing on the market and use of PFOA and APFO. RPS Advies, Delft, The Netherlands for European Commission Enterprise and Industry Directorate-General. Website Report.pdf
- ^ Association of State and Territorial Solid Waste Management Officials (ASTSWMO), 2015. Perfluorinated Chemicals (PFCs): Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) Information Paper. Remediation and Reuse Focus Group, Federal Facilities Research Center, Washington, D.C. Free download from: US EPA Report.pdf
- ^ Renner, R., 2001. Growing Concern Over Perfluorinated Chemicals. Environmental Science and Technology, 35(7), pp. 154A-160A. DOI: 10.1021/es012317k Open access article.
- ^ Fricke, M. and Lahl, U., 2005. Risk Evaluation of Perfluorinated Surfactants as Contribution to the current Debate on the EU Commission’s REACH Document. Umweltwissenschaften und Schadstoff-Forschung (UWSF), 17(1), pp. 36-49. DOI: 10.1007/BF03038694
- ^ Skutlarek, D., Exner, M. and Färber, H., 2006. Perfluorinated Surfactants in Surface and Drinking Waters. Environmental Science and Pollution Research International, 13(5), pp. 299-307. DOI: 10.1065/espr2006.07.326 Free download from: ResearchGate
- ^ Chemours, 2010. The History of Teflon Fluoropolymers. Website
- ^ Choi, D.G., Jeong, J.H., Sim, Y.S., Lee, E.S., Kim, W.S. and Bae, B.S., 2005. Fluorinated Organic− Inorganic Hybrid Mold as a New Stamp for Nanoimprint and Soft Lithography. Langmuir, 21(21), pp. 9390-9392. DOI: 10.1021/la0513205
- ^ Rolland, J.P., Van Dam, R.M., Schorzman, D.A., Quake, S.R., and DeSimone, J.M., 2004. Solvent-Resistant Photocurable “Liquid Teflon” for Microfluidic Device Fabrication. Journal of the American Chemical Society, 126(8), pp. 2322-2323. DOI: 10.1021/ja031657y
- ^ Moody, C.A. and Field, J.A., 1999. Determination of Perfluorocarboxylates in Groundwater Impacted by Fire-Fighting Activity. Environmental Science and Technology, 33(16), pp. 2800-2806. DOI: 10.1021/es981355+
- ^ National Foam, no date. A Firefighter’s Guide to Foam. Website Report.pdf
- ^ US Environmental Protection Agency (USEPA), 2018. Fact Sheet: 2010/2015 PFOA Stewardship Program. Website
- ^ Darwin, Robert L. 2011. Estimated Inventory of PFOS-based Aqueous Film Forming Foam (AFFF). Fire Fighting Foam Coalition, Inc., Arlington, VA. Report.pdf
- ^ Denton, Charles, 2019. Expert Focus: US states outpace EPA on PFAS firefighting foam laws. Chemical Watch. Website
- ^ FAA Reauthorization Act of 2018. US Public Law No: 115-254 (10/05/2018). Website Report.pdf
- ^ 76.0 76.1 Schultz, M.M., Higgins, C.P., Huset, C.A., Luthy, R.G., Barofsky, D.F., and Field, J.A., 2006. Fluorochemical Mass Flows in a Municipal Wastewater Treatment Facility. Environmental Science and Technology, 40(23), pp. 7350-7357. DOI: 10.1021/es061025m Author Manuscript
- ^ Michigan Waste and Recycling Association (MWRA), 2019. Statewide Study on Landfill Leachate PFOA and PFOS Impact on Water Resource Recovery Facility Influent, Second Revision. Report.pdf
- ^ Bossi, R., Strand, J., Sortkjær, O. and Larsen, M.M., 2008. Perfluoroalkyl compounds in Danish wastewater treatment plants and aquatic environments. Environment International, 34(4), pp. 443-450. DOI: 10.1016/j.envint.2007.10.002 Free download from: Academia.edu
- ^ Lin, A.Y.C., Panchangam, S.C., Tsai, Y.T., and Yu, T.H., 2014. Occurrence of perfluorinated compounds in the aquatic environment as found in science park effluent, river water, rainwater, sediments, and biotissues. Environmental Monitoring and Assessment, 186(5), pp. 3265-3275. DOI: 10.1007/s10661-014-3617-9
- ^ Ahrens, L., Felizeter, S., Sturm, R., Xie, Z. and Ebinghaus, R., 2009. Polyfluorinated compounds in waste water treatment plant effluents and surface waters along the River Elbe, Germany. Marine Pollution Bulletin, 58(9), pp.1326-1333. DOI: 10.1016/j.marpolbul.2009.04.028 Author’s manuscript
- ^ 81.0 81.1 Hamid, H. and Li, L., 2016. Role of wastewater treatment plant in environmental cycling of poly- and perfluoroalkyl substances. Ecocycles, 2(2), pp. 43-53. DOI: 10.19040/ecocycles.v2i2.62 Open access article.
- ^ Higgins, C.P., Field, J.A., Criddle, C.S., and Luthy, R.G., 2005. Quantitative Determination of Perfluorochemicals in Sediments and Domestic Sludge. Environmental Science and Technology, 39 (11), pp. 3946 – 3956. DOI: 10.1021/es048245p
- ^ US Environmental Protection Agency (USEPA), 2020. Research on Per- and Polyfluoroalkyl Substances (PFAS). Website
- ^ Sepulvado, J.G., Blaine, A.C., Hundal, L.S. and Higgins, C.P., 2011. Occurrence and Fate of Perfluorochemicals in Soil Following the Land Application of Municipal Biosolids. Environmental Science and Technology, 45(19), pp. 8106-8112. DOI: 10.1021/es103903d
- ^ Lindstrom, A.B., Strynar, M.J., Delinsky, A.D., Nakayama, S.F., McMillan, L., Libelo, E.L., Neill, M. and Thomas, L., 2011. Application of WWTP Biosolids and Resulting Perfluorinated Compound Contamination of Surface and Well Water in Decatur, Alabama, USA. Environmental Science and Technology, 45(19), pp. 8015-8021. DOI: 10.1021/es1039425
- ^ Blaine, A.C., Rich, C.D., Hundal, L.S., Lau, C., Mills, M.A., Harris, K.M. and Higgins, C.P., 2013. Uptake of Perfluoroalkyl Acids into Edible Crops via Land Applied Biosolids: Field and Greenhouse Studies. Environmental Science and Technology, 47(24), pp.14062-14069. DOI: 10.1021/es403094q Free download from: US EPA
- ^ Blaine, A.C., Rich, C.D., Sedlacko, E.M., Hundal, L.S., Kumar, K., Lau, C., Mills, M.A., Harris, K.M. and Higgins, C.P., 2014. Perfluoroalkyl Acid Distribution in Various Plant Compartments of Edible Crops Grown in Biosolids-Amended Soils. Environmental Science and Technology, 48(14), pp. 7858-7865. DOI: 10.1021/es500016s Free download from: ResearchGate
- ^ Washington, J.W., Yoo, H., Ellington, J.J., Jenkins, T.M., and Libelo, E.L., 2010. Concentrations, Distribution, and Persistence of Perfluoroalkylates in Sludge-Applied Soils near Decatur, Alabama, USA. Environmental Science and Technology, 44(22), pp. 8390-8396. DOI: 10.1021/es1003846 Free download from: ResearchGate
- ^ Oliaei, F., Kriens, D. and Weber, R., 2010. Discovery and investigation of PFOS/PFCs contamination from a PFC manufacturing facility in Minnesota—environmental releases and exposure risks. Organohalogen Compd, 72, pp. 1338-1341.
- ^ Minnesota Department of Health (MDH), 2020. Perfluoroalkyl Substances (PFAS) Sites in Minnesota. Website
- ^ Busch, J., Ahrens, L., Sturm, R. and Ebinghaus, R., 2010. Polyfluoroalkyl compounds in landfill leachates. Environmental Pollution, 158(5), pp.1467-1471. DOI: 10.1016/j.envpol.2009.12.031
- ^ Eggen, T., Moeder, M. and Arukwe, A., 2010. Municipal landfill leachates: A significant source for new and emerging pollutants. Science of the Total Environment, 408(21), pp. 5147-5157. DOI: 10.1016/j.scitotenv.2010.07.049
- ^ Allred, B. M., Lang, J. R., Barlaz, M. A., and Field, J. A., 2015. Physical and Biological Release of Poly- and Perfluoroalkyl Substances (PFAS) from Municipal Solid Waste in Anaerobic Model Landfill Reactors. Environmental Science and Technology, 49(13), pp. 7648-7656. DOI: 10.1021/acs.est.5b01040
- ^ 95.0 95.1 95.2 Lang, J.R., Allred, B.M., Field, J.A., Levis, J.W. and Barlaz, M.A., 2017. National Estimate of Per- and Polyfluoroalkyl Substance (PFAS) Release to U.S. Municipal Landfill Leachate. Environmental Science and Technology, 51(4), pp. 2197-2205. DOI: 10.1021/acs.est.6b05005
- ^ Lang, J.R., Allred, B.M., Peaslee, G.F., Field, J.A., and Barlaz, M.A., 2016. Release of Per-and Polyfluoroalkyl Substances (PFASs) from Carpet and Clothing in Model Anaerobic Landfill Reactors. Environmental Science and Technology, 50(10), pp. 5024-5032. DOI: 10.1021/acs.est.5b06237
- ^ Ahrens, L., Shoeib, M., Harner, T., Lane, D.A., Guo, R. and Reiner, E.J., 2011. Comparison of Annular Diffusion Denuder and High volume Air Samplers for Measuring Per- and Polyfluoroalkyl Substances in the Atmosphere. Analytical Chemistry, 83(24), pp. 9622-9628. DOI: 10.1021/ac202414w Free download available from: InforMEA
- ^ Benskin, J.P., Li, B., Ikonomou, M.G., Grace, J.R. and Li, L.Y., 2012. Per-and Polyfluoroalkyl Substances in Landfill Leachate: Patterns, Time Trends, and Sources. Environmental Science and Technology, 46(21), pp.11532-11540. DOI: 10.1021/es302471n
- ^ Clara, M., Scharf, S., Weiss, S., Gans, O. and Scheffknecht, C., 2008. Emissions of perfluorinated alkylated substances (PFAS) from point sources - identification of relevant branches. Water Science and Technology, 58(1), pp. 59-66. DOI: 10.2166/wst.2008.641 Open access article.
- ^ Trier, X., Granby, K. and Christensen, J.H., 2011. Polyfluorinated surfactants (PFS) in paper and board coatings for food packaging. Environmental Science and Pollution Research International, 18(7), pp. 1108–1120. DOI: 10.1007/s11356-010-0439-3
- ^ Fujii, Y., Harada, K.H. and Koizumi, A., 2013. Occurrence of perfluorinated carboxylic acids (PFCAs) in personal care products and compounding agents. Chemosphere, 93(3), pp. 538-544. DOI: 10.1016/j.chemosphere.2013.06.049
- ^ Organisation for Economic Cooperation and Development (OECD), 2013. Synthesis paper on per‐ and polyfluorinated chemicals (PFCs). OECD Environment Directorate/UNEP Global PFC Group. Website Report.pdf
- ^ US Environmental Protection Agency (USEPA), 2016. Drinking Water Health Advisory for Perfluorooctane Sulfonate (PFOS), EPA Document Number: 822-R-16-004. Office of Water, Health and Ecological Criteria Division, Washington, DC. Website Report.pdf
- ^ Björklund, J.A., Thuresson, K. and De Wit, C.A., 2009. Perfluoroalkyl Compounds (PFCs) in Indoor Dust: Concentrations, Human Exposure Estimates, and Sources. Environmental Science and Technology, 43(7), pp. 2276-2281. DOI: 10.1021/es803201a
- ^ Nilsson, H., Kärrman, A., Rotander, A., van Bavel, B., Lindström, G., and Westberg, H., 2013. Professional ski waxers' exposure to PFAS and aerosol concentrations in gas phase and different particle size fractions. Environmental Science: Processes and Impacts, 15(4), pp. 814-822. DOI: 10.1039/C3EM30739E
- ^ Kwok, K.Y., Yamazaki, E., Yamashita, N., Taniyasu, S., Murphy, M.B., Horii, Y., Petrick, G., Kallerborn, R., Kannan, K., Murano, K. and Lam, P.K., 2013. Transport of Perfluoroalkyl substances (PFAS) from an arctic glacier to downstream locations: Implications for sources. Science of the Total Environment, 447, pp. 46-55. DOI: 10.1016/j.scitotenv.2012.10.091